1.
Static
Electricity
a. Ions, as you recall, are charged atoms. In nature, where do you find ions?
Ions are found just about everywhere: in the solutions that make up oceans and rivers, in many of the minerals that make up the earth’s crust, in lightning and in the upper atmosphere, the so-called ionosphere.
b. When heterogeneous or other large objects gain or lose electrons, we say they are statically charged. But basically they share the same characteristics with ions:
|
Static
Charge |
Characteristic |
|
- |
Too many electrons (electrons>protons) |
|
+ |
Not enough electrons (protons >electrons) |
|
0
(neutral) |
# of protons = # of electrons |
The main difference between ions and objects that are
statically charged is the number of electrons transferred. For
instance, if we consider sodium chloride (table salt), which consists of ions,
then every sodium atom in the salt has lost an electron, and every chlorine atom
has gained an electron. But a glass rod that has just been rubbed with fur has
lost some electrons to the fur, but it is only some of the surface molecules
that have lost electrons to some of the fur molecules.
c. The rules of attraction and repulsion that applied to ions also apply to objects that have gathered static electricity:
|
Charges |
How They Behave
Towards Each Other |
|
+ , - |
Attraction |
|
+ , + |
Repulsion |
|
- , - |
Repulsion |
|
- , neutral |
Attraction |
|
+ , neutral |
Attraction |
|
neutral and neutral |
Nothing |
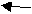
 Example 1
Example 1
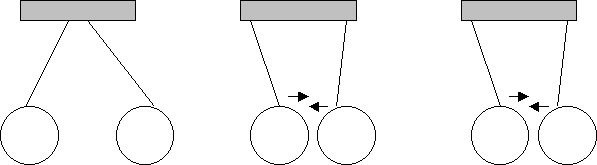
W Q Q R W R
What can you conclude about the charges of W, Q and R?
W and Q are of identical charges (since they repel). If W and Q are both (-) then R is (+)
If W and Q are both (+) then R is (-)
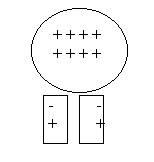
d. On a dry day, rub a balloon against your hair. Then press it gently against a wall or place it near small bits of paper. What happens? Why?
Assuming that the balloon has lost electrons to your hair, the balloon
now has a deficit of electrons. So it has a positive charge. The neutral bits of
paper will be attracted to the balloon because some of their electrons will, in
response to the (+) charge, move toward the surface and attract themselves to
the balloon. If the air is dry enough, and the pieces of paper small enough,
they will literally jump up and stick to a balloon placed a few inches above
them.
A few
basic rules:
(1) When
we draw a balloon with lots of +’s inside, we are not suggesting
that the balloon only consists of positives. It has lost some negatives so that
it now has more positives than negatives.
(2) Also, note
that we had electrons moving in the paper. In a previous discussion, electrons
moved out of the glass. In electrostatics, electrons move; positives do not
jump from one body to another.
(3) These
tricks only work when the air is dry. Water tends to neutralize
electrostatically charged materials. Smoke has a similar
effect.

Here a balloon is rubbed against a person’s hair. Because of the electron transfer, the hair and the balloon are of opposite charges and attract one another.
e. A student brings a negatively charged glass rod toward the knob of an electroscope. The electroscope consists of a metal knob attached to a long rod that slips through a hole in a rubber stopper, and at the end of the rod there are two thin sheets of silver referred to as “leaves”.
What do you think will happen if she comes close to the knob but never touches it?
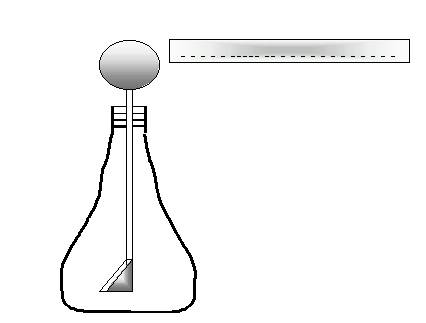
Electrons will be repelled by the (-)’s in the
rod, and they will seek refuge in the leaves, which are as far as the
electrons
can get from the rod. But that will make both leaves
negative, and they’ll repel. Meanwhile, the sphere will have
a
deficit of electrons. It develops a temporary positive charge, which will disappear as soon as the rod is moved away.
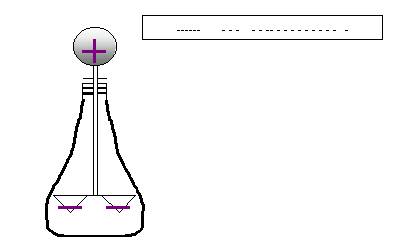
If the rod comes too to the rod or if it actually touches
it, electrons actually leak into the electroscope,
rendering both the sphere and leaves negatively-charged.