Solutions to Radioactivity
1. a) alpha
b) positron
2. a) 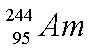
b) 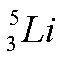
3. a) 15N
+ 1H à 4He
+ 12 C; check that atomic numbers balance: 7 + 1 = 2 + 6
b) 12C + 1H à 13N
+ g
13C
+ 1H à 14N
+ g
4. a) fission; we are
getting smaller nuclei
b) fusion; we are
getting a bigger hydrogen nucleus
5. a) ![]()
b) ![]()
c) ![]()
d) 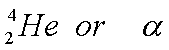
6. Compared to chemical changes, nuclear reactions release a lot more energy; they involve changes in the nucleus; they don't always preserve protons or neutrons; they don't conserve mass; and with the exception of X-rays, their electrons and shells don't change.
7. We had
shown on page 68 that for every mole of 2H fused, 1.65 X 109
kJ were released. (This was done by simply by
(1)Multiplying the
mass lost per 2H atom by Avogadro’s number = mass lost per reacting
mole
(2)Converting
to kg
(3)Using E = mc2,
and converting J to kJ
Now we’ll use that answer to get the energy released
for 28 g.
28g of 2H / 2 g/mole = 14 moles 2H
14 moles 2H (1.65 X 109 kJ/mole)
= 2.31 X 1010 kJ