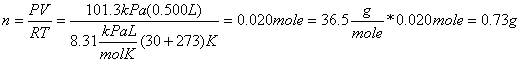
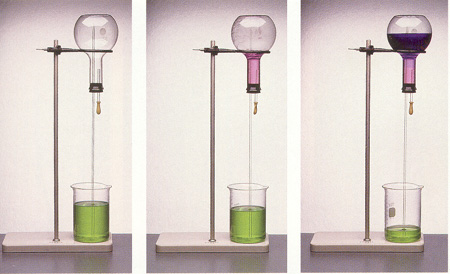
(1)....................................................... (2)......................................................... (3)
(1) The inverted Erlenmeyer flask was originally filled with air. The air was then displaced by HCl gas. There are now two glass tubes inserted in the rubber stopper. The first one is sealed with a bulb containing about 3 mL of water. The longer tube is connected to a beaker filled with water containing the indicator methyl purple along with a bit of base.
(2) and (3) The bulb is squeezed, which forces the water into the flask. Immediately, the green water goes up through the tube, but the water turns purple as it enters the flask.
Why is there a sucking effect? Why is there a colour change?
HCl is very soluble in water. At 30oC, 67.3 g of HCl dissolve in 100 mL of water. Proportionally up to 2 g can dissolve in the 3 mL that we squeezed into the flask. Did we even have 2 g of HCl in the 500 ml flask? Using PV= nRT, we can calculate the actual amount of HCl in the flask, assuming that it displaced most of the air originally in there:
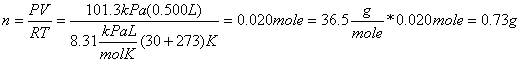
So clearly 3 mL of water is sufficient to dissolve all of the HCl gas. But since the gas is dissolved in the water, this leaves us with very little pressure inside the flask. Meanwhile atmospheric pressure pushes down on the green indicator and forces it up the glass tube and into the flask.
Why the colour change?
When HCl dissolves in water, it creates an acid:
HCl(g) + H2O(l) --> H3O+1(aq) + Cl-1(aq)
Or more simply: HCl(g) --> H+1(aq) + Cl-1(aq)
Remember the methyl purple was originally green because the water contained a bit of OH-1, but when it encounters the acidified water, the different pH creates the purple colour.
References: Merck Index.Twelfth Edition, 1996.
Harris, D.G. Quantitative Chemical Analysis 3rd Edition. Freeman, 1991.