the replacement of 2 percent of energy from trans fat with energy from unhydrogenated, unsaturated fats would reduce risk of coronary heart disease by 53 percent (95 percent confidence interval, 34 to 67; P<0.001)It's also common knowledge that a portion of the food industry responded to the new limitations by turning to palm oil as a substitute. So-called palm oil is actually a solid at sub-tropical temperatures because it melts at 33 to 39 oC. The reason for the melting point range is that palm oil like most triglycerides in food is not a single compound but a mixture of similar ones. But to create that trans-fat like texture the melting point is too high. So what's the solution?
First a quick review of triglyceride chemistry. A triglyceride is basically an ester, formed in nature by the reaction of fatty acids and glycerol.
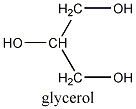
Here is a general formula for a triglyceride, whose properties will vary depending on the alkyl groups (R1, R2 and R3: highlighted in red). The glycerol skeleton is highlighted in blue.
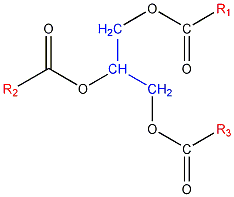
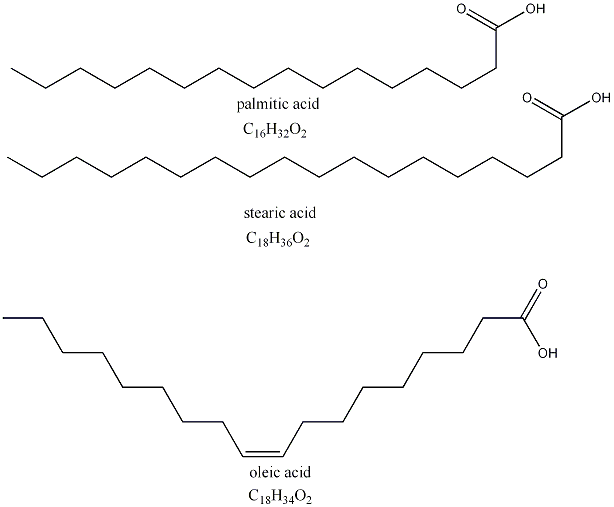
The R1, R2 and R3 groups are basically fatty acids minus the -CO2 group that's already represented in the general structural formula. Here are three examples of fatty acids.
Cocoa butter is a triglyceride obtained from the cocoa bean, and it is used to make chocolate. When mixed with the right ingredients, it's delicious. In addition, the fairly constant combination of fatty acids found in the beta form give this triglyceride another desirable property: a sharp melting point of 35 to 37oC, which coincides with the average range of human mouth temperatures. But cocoa butter is expensive, so in the past some of it was substituted with partially hydrogenated oils. Once anti-trans fat laws were passed, manufacturers of inexpensive chocolate could not simply use palm oil, whose melting point is not sharp enough. So what they did instead is rely on the interesterification of triglycerides, one of several methods of "modifying an oil". Eighty percent of cocoa butter triglycerides have palmitic and stearic acids in the R1 and R3 positions with oleic acid in the R2 slot. To create an impostor molecule from palm oil, a stearic acid residue is introduced at the R1 and R3 positions, where it's normally absent.
There are different ways of interesterifying. The superior method relies on enzymes because it leaves the R2 position unchanged. The catalyst that creates a greater hodge-podge of products is sodium methoxide. In either case, we don't exactly have the equivalent of a "Nurse's Study" to investigate the health impact of these molecules that are being included in foods.
Look for modified palm oil in the average candy bar and especially in cheap Easter chocolate, where it is the second most common ingredient after sugar. It is also found in albeit smaller quantities(3 to 5%) in several versions of trans fat -free margarine.
REFERENCES
http://palmoil.com/useful_info/dd Information about palm oil from the industry
http://www.nejm.org/doi/full/10.1056/NEJM199711203372102 The Nurse's Study
http://www.lsbu.ac.uk/biology/enztech/interester.html More details on interesterification
http://www.canada.com/topics/news/story.html?id=fd113bdf-6686-496a-8161-06a85546a1b8A General article on modified fats