
Morphine, C17H19NO3, is the most abundant of opium’s 24 alkaloids, accounting for 9 to 14% of opium-extract by mass.
Named after the Roman god of dreams Morpheus, who also became the god of slumber, the drug morphine, appropriately enough, numbs pain, alters mood and induces sleep. Less popular and less mentioned effects include nausea, vomiting and decreased gastrointestinal motility. (It’s a great constipator, and in Guerin’s painting, Isis is perhaps bringing Morpheus a laxative.) Morphine and its related synthetic derivatives, known as opioids, are so far unbeatable at dulling chronic or so-called “slow” pain, but unfortunately they are all physically addictive. During the American Civil War, 400 000 soldiers became addicted to morphine. Both morphine and its hydrated form,C17H19NO3.H2O, are sparingly soluble in water. In five litres of water, only one gram of the hydrate will dissolve. For this reason, pharmaceutical companies produce sulphate and hydrochloride salts of the drug, both of which are over 300 times more water-soluble than its parent molecule. Whereas the pH of a saturated morphine hydrate solution is 8.5, the salts are acidic.
Since they derive from a strong acid but weak base, they are both at about pH = 5; consequently in the manufacturing stage, morphine salts are mixed with small amounts of NaOH to make them suitable for injection.
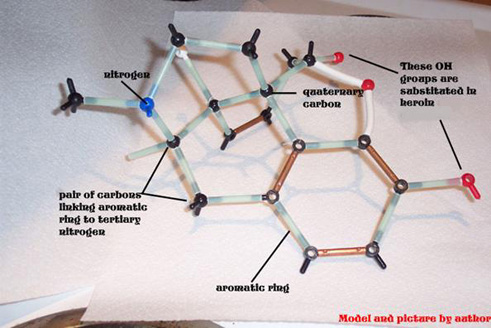 The three dimensional structure of morphine is fascinating. It consists of five rings, three of which are approximately in the same plane. The other two rings, including the nitrogen one, are each at right angles to the other trio.
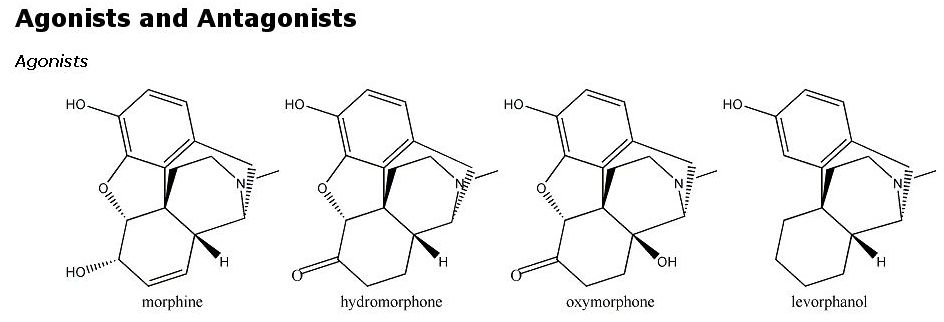
Opioid analgesics, including morphine, codeine, levorphanol, heroin and structurally less similar drugs such as meperidine all have an aromatic ring and a quaternary carbon atom linked to a tertiary amine group by two other carbon atoms.
The three dimensional structure of morphine is fascinating. It consists of five rings, three of which are approximately in the same plane. The other two rings, including the nitrogen one, are each at right angles to the other trio.
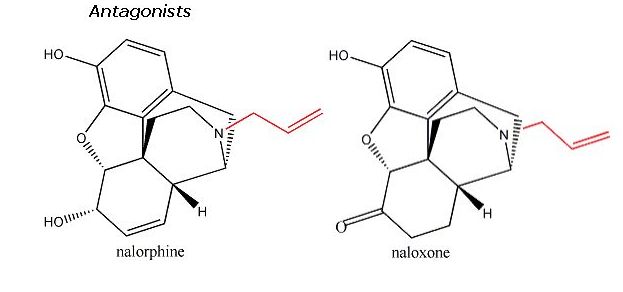
Opioid analgesics, including morphine, codeine, levorphanol, heroin and structurally less similar drugs such as meperidine all have an aromatic ring and a quaternary carbon atom linked to a tertiary amine group by two other carbon atoms. This is known as the morphine rule, but it should be pointed out that all of the opioids below also have a methyl group attached to a nitrogen atom. Substitute morphine’s methyl group with a propenyl group, and you create nalorphine, an antagonist which counters morphine’s effects. Similarly, hydromorphone, a ketone version of morphine five times more powerful than its parent molecule becomes the antagonist hydromorphone by the same substitution of a CH3 with a CH2CH=CH2group. More generally, morphine’s b-phenylethylamine unit (essentially what I just described minus the quaternary carbon atom) is also found at the tail-end of enkephalin molecules.
 Enkephalins are smaller versions of endorphins, which are produced naturally in the brain, pituitary and other tissues, where they act very much like opium’s molecules.
Morphine acts on a specific receptor of nerve cells. More specifically many such receptors are found in the spinal cord’s substantia gelatinosa, a region where pain signals are first processed. The architecture of the morphine receptor is what dictates the morphine rule. There is a flat part that binds to the aromatic ring, a cavity that attracts the two carbon atoms and an anionic site that accommodates the tertiary nitrogen atom. When morphine or another agonist binds to the receptor, the cell membrane’s affinity for sodium ion changes. This eventually reduces the release of neurotransmitters from the affected neurons.
Enkephalins are smaller versions of endorphins, which are produced naturally in the brain, pituitary and other tissues, where they act very much like opium’s molecules.
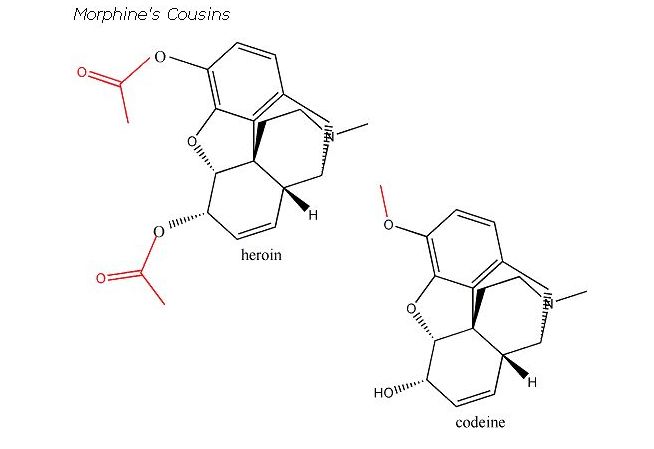
Morphine acts on a specific receptor of nerve cells. More specifically many such receptors are found in the spinal cord’s substantia gelatinosa, a region where pain signals are first processed. The architecture of the morphine receptor is what dictates the morphine rule. There is a flat part that binds to the aromatic ring, a cavity that attracts the two carbon atoms and an anionic site that accommodates the tertiary nitrogen atom. When morphine or another agonist binds to the receptor, the cell membrane’s affinity for sodium ion changes. This eventually reduces the release of neurotransmitters from the affected neurons. Investigators learned about morphine’s mode of action by applying it and other opiates (including enkephalin) to guinea-pig intestines. (What else was gong to serve as the guinea pig for their experiments?) In the presence of antagonists, Na+ affinity was restored and intestinal contractions which had dropped precipitously shot up again. Not all opiates are created equally. Codeine is only 0.1 % as potent as morphine in binding to receptors. But because codeine is converted to morphine by the liver (all that has to happen is that the OCH3 group gets replaced by OH) it becomes 20% as strong as the latter overall. In the late 1800’s, the Bayer company, in hoping to come up with a non-addictive pain killer, tried to acetylate both of morphine’s hydroxyl groups. After all, this was in a way similar to how they had converted salicylic acid into aspirin.
But embarassingly, Bayer invented heroin in the process.
The 1914 Harrison Narcotics Tax Act in the United States made possession of morphine a criminal act. Forensic scientists exploit group characteristic property of morphine and its related compounds: a peculiar reaction with Mecke's (or Lafon's) reagent. The reagent consists of selenious acid(H2SeO3)in concentrated sulfuric acid (H2SO4). If morphine and the reagent react, the following colours appear: green, then quickly a greenish blue, changing to blue, next slowly to bluish green with a yellow-brown edge, then finally olivaceous green. This is one of a variety of color tests that are used. Most, however, are presumptive tests, meaning that they suggest the presence of opiates but don't convincingly prove it. Final confirmation is obtained through instrumentation, either gas chromatography-mass-spectrometry or by Fourier Transform Infrared Spectroscopy. NMR has also been used to identify heroin.
PRINTED REFERENCES
Schnell, B.R. and al. Compendium of Pharmaceuticals and Specialties. Canadian Pharmacists Association. 1999.
LeCouteur P. and Burreson J. Napoleon’s Buttons: How 17 Molecules Changed History. Penguin Putnam. 2003 Budavari, Susan. The Merck Index, 12th edition. Merck. 1996
Atkins, P.W. Molecules. Scientific American Library. 1987
Snyder, Solomon H. “Opiate Receptors and Internal Opiates.” Scientific American. p44-56. March 1977.
ONLINE REFERENCES
http://aafs.micronexx.com/PDF/JOFS/JFS4550963/JFS4550963.pdf
http://webbook.nist.gov/cgi/cbook.cgi?ID=C57272&Units=SI&Mask=80#IR-Spec
http://www.chamisamesa.net/morphine.html
http://en.wikipedia.org/wiki/Morphine
http://www.druglibrary.org/schaffer/heroin/opiates.htm
http://www.ch.ic.ac.uk/rzepa/mim/drugs/html/morphine_text.htm