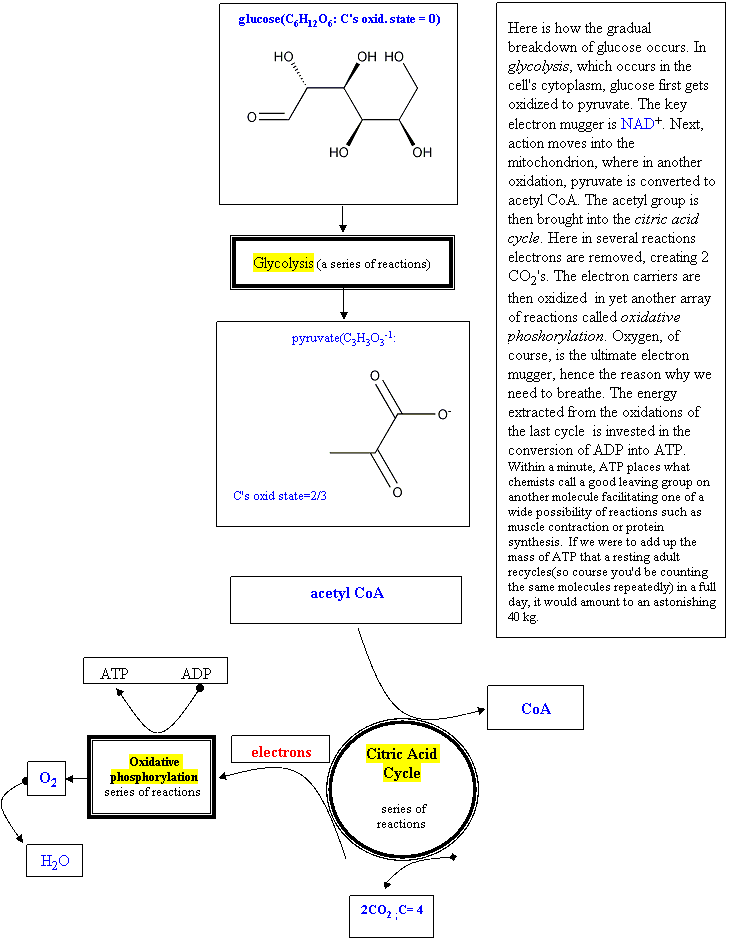
Cellular Respiration: An Assembly of Redox Reactions
Glucose is a universal fuel for living cells. But there is a world of difference between the simple way an automobile engine uses gasoline as a fuel and the set of reactions that a cell relies on to obtain energy from glucose. To oxidize a mixture of octanes, an engine lets in oxygen and creates the necessary heat from the pistons' pressure and a spark. Then the hot expanding gases of combustion, mainly steam and carbon dioxide, push back on the pistons, moving the crankshaft and eventually turning the wheels. A living cell, on the other hand, does not have access, to the amount of activation energy that would be necessary to burn glucose in one or two steps. Nor does it want to. The reaction is too exothermic, and the heat would just destroy the rest of the delicate cellular machinery.
So over the course of evolution, matter has found ways of slowly extracting energy from fuel molecules and investing part of that energy in assembling special molecules that could then facilitate non-spontaneous reactions. To do otherwise would be analogous to attempting to travel across a mountain by first sprinting up and then jumping off the nearest cliff.
Printed References
Stryer, Lubert Biochemistry.Fourth Edition. Freeman. 1988
Chernow and al.(editors).Columbia
Encyclopedia. 1993
Online References
J. Stein
Carter
This well-renowned site includes some neat ATP calculations towards the bottom
of the page.
Comments: euva@retired.ca
Copyright ©2004
A violation of sig figs!!