Extra Practice
Problems for 2010 Pretest 1.1
1.Extra Charles Law Problem
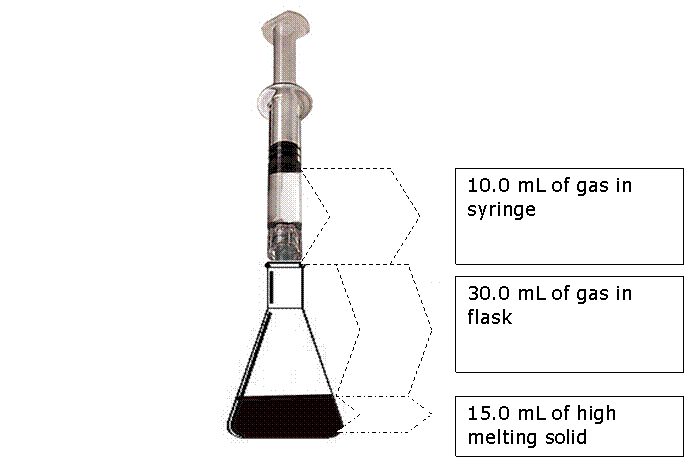
If the all the gas in the syringe and connected glass flask is heated from 5.00 o C to 10.0 o C at constant pressure, what will be the volume of gas in the syringe?
Answer:
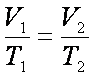
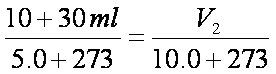
V2= 40.72ml (Total)
Syringe will read 40.72 ml – 30.00 ml = 10.7 ml. (3 SF)Notice that we ignore the volume of the solid. Never place volumes of solids and liquids into gas laws!
2. A student did some calculations using Charles’ Law and expected to obtain a final volume of 22.4 L. Instead the volume (which was measured in a cylinder with a piston) was actually 21.4 L. Aside from experimental error, what gas behaviour could account for a smaller than expected volume?
Answer
Not all gases are ideal. Some so-called “ real” gases have attractions between gas molecules, leading to a lower than expected volume.
3. Why do liquids expand when heated?
The heat causes extra rotational motion, which gets the molecules to expand .