Chemistry 534
Pretest 2.1
1. A student was working
on her pretest. Meanwhile 1000 kJ from the slab of cheesecake she ate earlier
was used for basic bodily functions, and 600 kJ were used to store vital course-related
information. If the slab of cheesecake contained 2000 kJ in all, how much
energy was lost in the form of heat? Why?
2000 kJ = 600 kJ + 1000 kJ + x (based on
the conservation of energy)
x = 400 kJ.
2. Draw two H2O2 molecules. Label all intermolecular and intramolecular bonds.
![]()
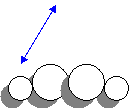
An intermolecular attraction
occurs between two molecules, as shown by the two headed arrow. Each H2O2
also has 3 intramolecular attractions because
there are three bonds per molecule.
3. a. Draw a
reaction profile for an exothermic reaction.
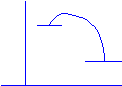
E(kJ)
Reaction
progress
b.
b.
Do the same for
an endothermic reaction.
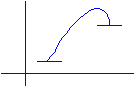
E(kJ)
Reaction
progress
4. When nitric acid, sulfuric acid and glycerin are mixed, they
react to form a new compound and a great deal of heat is released. Where is
this heat coming from?
From the
excess enthalpy of nitric acid, sulfuric acid and glycerin. More specifically, the energy comes mostly from the intramolecular bonds of the reactants mentioned.
5. Which of the following
defines enthalpy?
|
A) |
The
energy absorbed or released during a chemical reaction |
|
B) |
The
change in potential energy that results from a chemical or physical change |
|
C) |
The
energy required to start a chemical reaction |
|
D) |
The
internal energy stored in a substance during its formation |
Answer (D) (A) and (B) are definitions of the change
in enthalpy (DH). (C) is
the definition of activation energy.
Note that enthalpy can
also be defined as the sum of potential and kinetic energies of a substance.
6. Which of the following changes are
exothermic?
1. Logs
burning on a camp-fire
2. The
action of an ice-salt mixture used to freeze ice cream
3. Laundry
that dries on a clothesline
4. A
chicken roasting
5. A bolt
of lightning
Answer: 1,
2 and 5.
7. This was , as you can see, #10 on the
June 2001 exam:
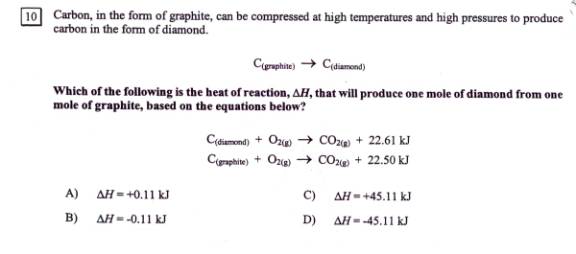
Reverse eq(1):
CO2+ 22.61 kJ à C(diamond) + O2 DH = 22.61 ( if heat is a “reactant”, DH = +)
Keep eq(2) as it is:
C(graphite)
+ O2à CO2+
22.50 kJ DH = -22.50 ( heat is released)
Sum: C(graphite) à C(diamond) DH = 0.11 kJ
8. Some automobiles and buses are equipped to burn
propane gas, C3H8, as a fuel.
The
complete combustion of propane is shown by the following chemical equation:
![]()
Given the following heats of formation.
H2(g)
+ ![]() O2(g) ® H2O(g) DH = -242.0
O2(g) ® H2O(g) DH = -242.0 ![]()
C(s) + O2(g) ® CO2(g) DH = -393.5 ![]()
What is the heat of formation of propane?
3
C(s) + 4 H2(g) ® C3H8(g) DH = ?
Solution:
|
|
3 C(s) + 4 H2(g) ® C3H8(g) |
DH = ? |
|
Reverse the
«combustion of propane» equation and add to it the heats of formation of CO2(g)
and H2O(g) |
||
|
(Reverse) |
4 H2O(g)
+ 3 CO2(g) ® C3H8(g) + 5
O2(g) |
DH = + 2044.5 |
|
(mult ´ 4) |
4 H2(g) + 2 O2(g) ® 4 H2O(g) |
DH = - 968 |
|
(mult ´ 3) |
3 C(s) + 3 O2(g) ® 3 CO2(g) |
DH = - 1180.5 |
|
|
||
|
|
3 C(s) + 4 H2(g) ® C3H8(g) |
DH = - 104 |
Answer : The heat of formation of propane is - 104 ![]() .
.
9. A bathtub contained 200.0 kg of water. The water's temperature increased from 20.4
to 38.4 oC when it absorbed the heat from
the combustion of of 420.0 g of C3H6
. Find the molar DH for the combustion
of C3H6.
(Ans: DH =-1.51 X 103 kJ/mole)
10. 0.20 moles of H2SO4(aq) were completely neutralized by
aqueous NaOH. The resulting 600.0 ml of mixture
experienced a 15 degree increase in temperature. What is the molar heat of
neutralization of aqueous NaOH? Density = 1.00
g/ml)
Q = mcDT
=600.0ml(1.00g/ml)(4.19J/(g oC))(15)oC
=
38011.68000J
=38.01168 kJ
DH =-Q
=-38.01168 kJ
Since the ratio is 2:1 2 NaOH
+ H2SO4 à Na2SO4
+ 2 H2O, 0.40 moles of NaOH reacted with 0.20 moles of H2SO4(aq).
DH/n
=-38.01168 kJ/0.40moles =-95kJ/mole NaOH
Flashback
1. What is the minimum number of litres
of O2 needed to completely burn 320. g of CH3OH if the reaction occurs at 150. kPa and 400. oC?
2 CH3OH(l) + 3 O2(g) à 2 CO2(g) + 4 H2O(l)
320 g of CH3OH/(32 g/ mole of CH3OH) = 10 moles of CH3OH
The ratio of O2(g) to CH3OH from the
equation is 3 to 2, so we need 1.5*10 = 15 moles of O2(g).
PV = nRT;
V = nRT/P = 15(8.31)(400 + 273)/150
= 559 L
2. Which 3 of the following factors influence the volume
occupied by a gas?(JUNE 2001)
__pressure ___temperature ___density __solubility __#
of moles
P,T and n
because V = nRT/P.