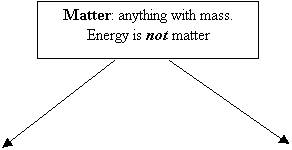
Classification
of Matter
Elements:
contain only one kind of atom. The chemical symbol only has one capital
letter Compounds:
contain two or more elements bonded together chemically. The chemical
formula contains two or more capital letters
Examples
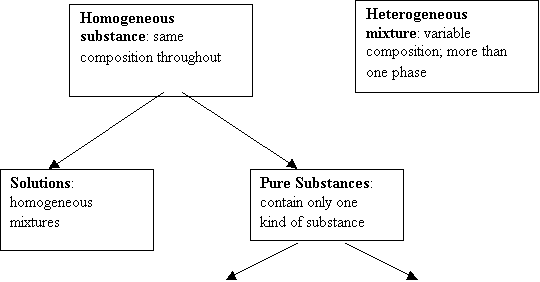
Heterogeneous
mixture: granite rock, banana
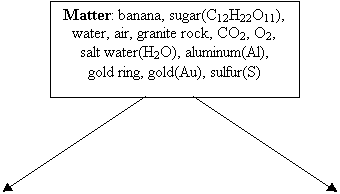
Solutions:
homogeneous mixtures: air, salt water, gold ring Pure Substances:
sugar(C12H22O11), water(H2O), CO2, O2,
aluminum(Al), gold(Au), sulfur(S)
![]()
![]()
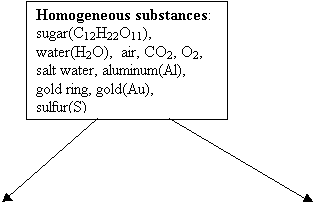
Elements: O2,
aluminum(Al), gold(Au), sulfur(S) Compounds:
sugar(C12H22O11), water (H2O),
CO2,
Elements
Versus Compounds
Just what is the difference between a compound and an element? There are over 114 known elements: these are listed in the periodic table, and they make up all matter in the universe: rocks, stars, dust and living beings. Each element is made up of only one kind of atom, meaning that each atom has a specific number of protons.
As mentioned, elements have to react chemically to form compounds. As atoms bond, energy is either released or absorbed. The reverse can also take place. When a compound decomposes, we often see its two components released, and if one of them is a gas, the leftover solid or liquid will weigh less than the original solid or liquid that decomposed.
Example: 2 HgO(s)-->
2Hg(s) + O2(g)
432 g --> 400 g + 32g
Also the original solid (HgO) and the product (Hg) are not the same colour. HgO is red and Hg is shiny and silvery. We have gas escaping (as suggested by the loss in solid mass: 432 vs. 400g) and a solid that is different from the original (difference in colour); the combination of these two observations suggest a chemical change.(see later notes)
If we had iodine going from a shiny solid to a purple gas, we would again observe an apparent loss in solid mass, but the solid left behind would not be of a different colour. Also in this physical change(sublimation) the iodine gas would turn back into shiny iodine crystals upon cooling. In the chemical reaction involving HgO, the oxygen gas escaping would not turn back into red HgO powder.