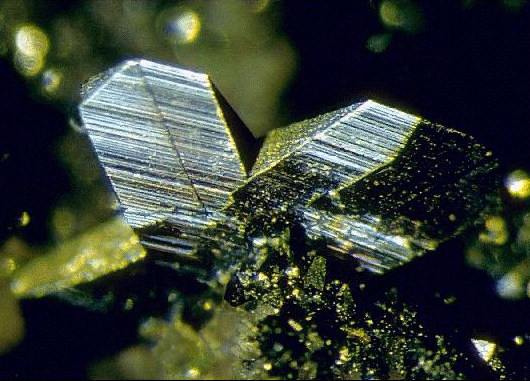
Pic from http://www.mineralatlas.com/mineral%20photos/M/marcasite2.jpg
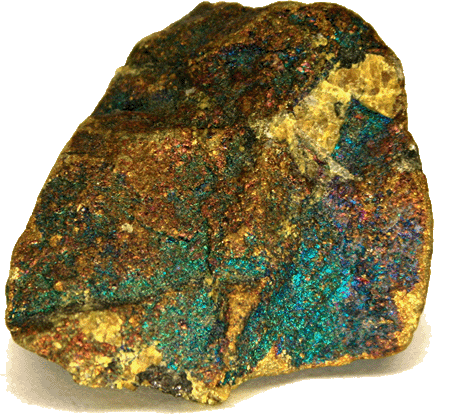
Pic from Wikipedia.

Pic from http://gwydir.demon.co.uk/jo/minerals/calcite.htm

Pic from http://www.gc.maricopa.edu/earthsci/imagearchive/realgar.jpg

Pic from author.

Pic from http://www.sedgwickmuseum.org/education/gallery.html

Pic from author.
(not really a mineral but I formed this common organic compound by slow crystallization)