Electrochemistry
Objective A: to identify the parts of an electrochemical cell and to describe their function.



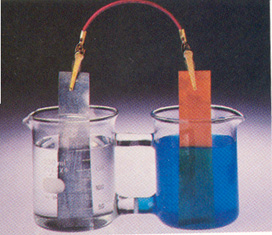
Observations:
Explanation:
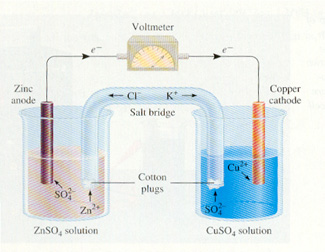
Zinc undergoes oxidation:
Zn--> Zn+2 + 2e
Since the metal turns into aqueous ions, this explains why the zinc strip shrinks. In the meantime, lost electrons flow toward the copper ions in the other beaker, lighting th ebulb in the process.
Electrons flow through the Cu strip where they attract the Cu+2 ions and reduce them.
Cu+2 + 2e --> Cu
The overall reaction is
Zn + Cu+2 --> Zn+2 + Cu
Zn = reducing agent; Cu+2 = oxidizing agent
Why a salt bridge?
Without the KNO3-salt bridge, excess positive charge(from Zn+2) would accumulate at the Zn anode and too much negative charge would accumulate at the Cu cathode (because Cu+2 is being consumed). Nitrate (NO3-1) from the salt bridge compensates for the additional (+'s) formed by Zn, while K+1 moves into the Cu+2 beaker and replaces lost (+'s).
Key Definitions
:|
Anode |
An electrode (an electrical conducting rod or strip) where oxidation occurs. |
|
Cathode |
An electrode where reduction occurs. |
|
Anion |
A negatively charged ion which is attracted to the anode (where (+) charges are formed) |
|
cation |
A positively charged ion which is attracted to the cathode (where electrons are reducing the (+) ions) |