Justify all your choices.
1. When zinc metal reacts with HCl, hydrogen gas is produced according to:
Zn(s) + 2 HCl(aq) --> ZnCl2(aq) + H2(g)
Which would INCREASE the rate of formation of hydrogen?
- Use powdered zinc instead of a solid piece of zinc.
- Increase the temperature of the acid.
- Increase the volume of the acid used.
- Increase the size of the reaction container.
- 1 and 2
- 1 and 4
- 2 and 3
- 3 and 4
2. The thermal decomposition of calcium carbonate (CaCO3) produces quicklime or CaO and CO2, according to
CaCO3 ---> CaO + CO2
A sample of calcium carbonate weighing 0.45 g was heated and reacted as shown above. After 3.00 minutes, the unreacted calcium carbonate was removed and found to weigh 0.31 g.
What was the average rate of reaction measured in moles of calcium carbonate reacted per minute?
- 4.7 X 10-2
- 1.0 X 10-3
- 1.4 X 10-3
- 4.7 X 10-4
3. Which of the following reactions will occur the most rapidly at room temperature?
- CH4(g) + 2 O2(g) --> CO2(g) + 2 H2O(g)
- 2 H2(g) + O2(g) --> 2 H2O(g)
- Ba+2(aq) + SO4-2(aq) --> BaSO4(s)
- Cu(s) + 2 Ag+1(aq) --> 2Ag(s) + Cu+2(aq)
4. The following energy distribution curve shows the threshold energy of two reactions labeled A and B.
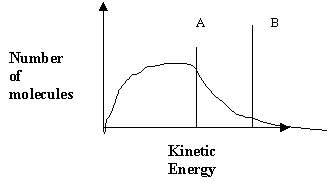
Which of the following statements are necessarily TRUE?
- Reaction A has a lower activation energy than reaction B.
- Of the two reactions, reaction A has a greater number of molecules with sufficient energy to react.
- Reaction A has a larger DH.
- An increase in temperature will only increase the rate of reaction A and not that of B.
- 1 and 2
- 1 and 4
- 2 and 3
- 3 and 4
5. Which of the following statements concerning molecular collisions are true?