Law
of Chemical Equilibrium
Since a reaction at equilibrium has fixed concentrations of products and reactants, we can calculate a constant for a given reaction at constant temperature.
For the generalized equation
††††††††††††††††††††††††††††††††††††††††††††††† aB + cD† ![]() †eF + gH
†eF + gH
††††††††††††††††††††††††††††††††††††††††††††††† 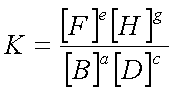 ,
,
where [ ] represents the equilibrium concentration in moles/L
Keep in mind: You only include
concentrations of aqueous and gaseous reactants/ products, not those of liquids
or solids.
Example 1
Given the following concentrations in moles/L at 748 C
CO2(g) + H2(g) ![]() CO(g) + H2O(g)
CO(g) + H2O(g)
0.00630†††††††††† 0.00630†††††††††† 0.00552†††††††††† ††† 0.00552
Write the equilibrium law expression, and then calculate the constant at that temperature.
 = 0.768
= 0.768
Example 2
Suppose 6.000 mol of F2 and 3.000 mol of H2 are mixed in a 3.000 L container to make HF . The equilibrium constant at a certain temperature is 1.15x10 2.
Calculate the equilibrium concentrations, given:
H2(g) + F2(g) †![]() 2 HF(g)
††
2 HF(g)
††
In such problems, it is very
important to distinguish between
(1)
the number of moles/L
initially found in the reaction vessel
(2)
the number of moles/L
that actually react or form
(3)
the number of moles/L
found at equilibrium
|
|
1
H2(g) |
1
F2(g) † |
2
HF(g) †† |
|
Initial # of moles |
3.000/3 = 1.000 |
6.000/3 = 2.000 |
0 (since it is not mentioned,we have to assume
there was none introduced. |
|
Moles/L reacting/forming |
x,
since we donít know how much reacts |
x,
since the ratio of† H2(g) to F2(g)
†in the equation is 1:
1 |
2x , since 2 moles of HF form for every 1 mole of† reacting H2. |
|
# of moles/L at equilibrium |
1-x
remain |
2-x
remain |
0 + 2x now exist. |
We write an equilibrium law
expression, and then substitute the equilibrium number of moles into that
expression. Donít forget to divide by the number of litres,
which in this example is 3.0 L.
![]()
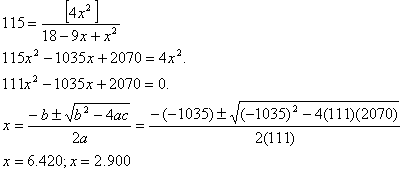
If we had only 1.00 mole/L of
hydrogen initially, ![]() †is the only sensible answer.
†is the only sensible answer.
Now we can wrap up the problem and
calculate the equilibrium concentrations:
