The Evolution of Elements
††††††††††† In the universeís original stars and in present suns that cannot attain core temperatures beyond 106 K, the main source of energy results from the proton-proton chain.
††††††††††† Four hydrogen nuclei initially fuse to produce two deuterium nuclei, two positrons and two neutrinos:
![]() †††††††††† (1)
†††††††††† (1)
†††††††††††
††††††††††† The big coefficient acts as a multiplier .The lower number next to each atomic symbol reveals the number of protons in each atom. The upper number(mass number) minus the number of protons reveals the number of neutrons.
†Notice that we originally have four protons
and no neutrons becoming only two protons and suddenly two neutrons, two
positrons (![]() )
and two neutrinos(
)
and two neutrinos(![]() ).
Two of the protons that disappeared have become neutrons. If we divide through
by 2:
).
Two of the protons that disappeared have become neutrons. If we divide through
by 2:
![]() †††††††††††††††††††††† (2)
†††††††††††††††††††††† (2)
A neutron has no charge but a mass unit of 1. The positron is a component of anti-matter. Like the electron it has very little mass but the positron bears a positive charge. When created, it soon meets an electron in the starís plasma; they annihilate each other and release energy. The neutrino is an even lighter particle than the electron, which even before being detected, was postulated to balance the amount of kinetic energy on both sides of the equation.
If we go back to equation (2), and remember that a proton consists of two up quarks and a down quark, and that the reverse combination makes up a neutron, than what is happening at a more fundamental level, is that an upquark is being converted into a down quark with the accompanying release of a positron and a neutrino.
After the reaction of equation (1), two different isotopes of hydrogen fuse to create a second isotope of helium. This leads to a small mass-loss which according to E = mc2 translates into an enormous amount of energy:
![]()
![]() (3)
(3)
Then
![]() †††† (4)
†††† (4)
Overall by combining (1), (3) and (4) we obtain:
![]()
In suns that benefit from the formation of elements from previous generations of stars, and if the core temperature exceeds 16 X 106 K, carbon acts as a catalyst:
![]()
![]()
![]()
![]()
![]()
![]()
Overall the reaction is the same as that of the proton-proton chain:
![]()
When hydrogen is depleted at the core of the star, radiative pressure decreases and gravity causes contraction. When temperature increases to 1 X 108 K, He begins to fuse and the star swells into a red giant:
![]()
![]()
![]()
When the core is left with only C and O, the star becomes a planetary nebula and ejects its outer shell. Eventually it becomes a white dwarf. Electron degeneracy pressure (Pauli exclusion principle prohibits two electrons from having the same quantum state.) prevents further contraction.
If the mass of the star was at least 4 solar masses prior to ejection, then:
![]()
![]()
![]()
If the star was at least 9 solar masses prior to ejection and if the T > 109 K:
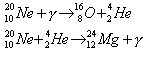
If T > 1.5 X 109 K, then
![]() , or
, or 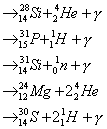
In a star of 25
solar-masses, temperatures reach 3 X 109 K, and for six months:
Overall we obtain:
![]()
With such a large mass,
electron degeneracy pressure is exceeded by gravity and protons and electrons
fuse to create a neutrons and neutrinos:
![]()
When mass from surrounding
layers converge on the extremely dense core of neutrons, an extremely violent
event known as a supernova (type II) occurs. Some of the ensuing energy
released is used to create a variety of both lower and higher isotopes.
First a nucleus must absorb
neutrons. An excess of neutrons will spark the conversion of a neutron to a
proton and an electron, thus increasing the atomic number.
![]()
In a main sequence star such
reactions are slow because of the low likelihood that a neutron will encounter
a nucleus, but a supernova implosion creates an abundance of neutrons needed
for neutron decay.
If a white dwarf has a
companion star, it may draw additional material to its surface, leading to
either a nova or if there is even more mass( more than 1.4 solar masses) drawn
a higher density and temperature leads to the fusion of carbon and oxygen into
iron. This happens uncontrollably; the violent event is known as a supernova
type I and it leads to a different array of isotopes from the ones produced in
a type II supernova.
![]()
![]() References:
References:
- Radiation and Radioactivity. Draganic
and al. CRC Press. 1990
- Cambridge Encyclopedia of Astronomy.
Greenberg and al. Prentice Hall
- Universe. Kaufmann. W.H. Freeman. 1985