Chemistry Exam Review 2
PART A. Circle the correct answer.
1. If the enthalpy of a particular reaction is negative, the reaction is:
a) exothermic b) endothermic c) synthesis d) spontaneous
2. Which of the following is FALSE as applied to the reaction ?
H2(g) àH(g) + H(g) DH = + 26 kJ
a) The DH means the reaction is exothermic.
b) Two moles of H gas contains more energy than one mole of H2 gas.
c) Weight for weight, H gas would be better fuel than H2 gas.
d) Heat is required to make H gas from H2 gas.
3. In a calorimeter, a base solution is added to 150 g of water and results
in the liberation of 9450 J of heat. Calculate the increase in temperature
of the water. (c for water is 4.19 J/[g. oC])
a)
4.2 o C b) 15 o C c) 26 oC d) 63 o C
4. The molar heat of reaction is __________.
a) the sum of the energies stored in a substance during its formation.
b) the quantity of heat absorbed or released during the reaction of a given amount of substance.
c) the amount of heat absorbed or released from one mole of a substance in a chemical reaction.
d) the quantity of energy involved in the formation of one gram of substance.
5. Identify the reaction(s) which is/are endothermic.
a) rubbing alcohol on your skin
b) ice melting
c) splashing after-bath on skin
d) all of the above
e) none of the above
6. Which of the following statements concerning molecular motion are false?
1. Translational motion of molecules is evident in the liquid state.
2. Rotational motion is movement of a molecule's atoms about its center.
3. As the temperature of a solid rises, the motion of the molecules increases.
4. In a solid, molecules vibrate faster when heat is removed.
a) 1 and 3
b) 1 and 4
c) 2 and 3
d) 2 and 4
7. Which statement(s) correctly describes the following reaction ?
CH4(g)
+ 2 O2(g) àCO2(g)
+ 2 H2O(g) DH = - 880 kJ/mol
I The reaction is endothermic.
II The temperature of the system drops.
III The combustion of 2 mole of CH4(g) absorbs 1760 kJ of energy.
IV The reaction is exothermic.
a) II , IV b) IV c) I d) II , III
PART B.. Show all work.
8. Given the following equations,
1/2 N2(g) + 3/2 H2(g) à NH3(g) DH = -47.7 kJ
H2O(g) à H2(g) + 1/2 O2(g) DH = +241.8 kJ
The burning of ammonia, NH3, is represented by the equation
4 NH3(g)
+ 3 02(g) à2 N2(g) + 6 H2O(g)
Calculate DH for the combustion of ammonia.
9. If one burns 3 g of ethane (C2H6) in a calorimeter containing 500 g of water. The initial temperature of the water was 25 oC . At the completion of the combustion, the thermometer reads 33 oC . Knowing that the specific heat of water is 4.2 J/g. oC, calculate the molar heat ( DH ) for ethane.
10. 300 g of water are originally at 20 C. A 100 g piece of tungsten originally at 90 C is added to the water. Show that the temperature change is almost negligible(very small).
(c for water is 4.19 J/g. oC; c for W (tungsten) = 0.032 J/g. oC)
11. The diagram below represents the reaction described by the equation:
CO2(g) + H2(g) àCO(g) + H2O(g)
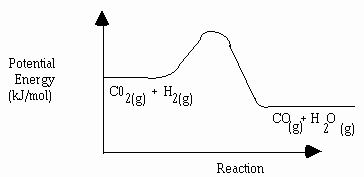
Use "a" to indicate the enthalpy change of reaction on the graph.
Indicate "b" to indicate activated complex on the graph.
Indicate "c" to indicate activation energy of the forward reaction on the graph.
Copyright ©2009
Created:April/6/1996; Updated:Dec/3/2009