Chemistry January Exam Review 3
(The
questions from this review were written by J. Daskalakis.)
PART A. Circle the BEST answer.
1.If a student increases the temperature of the reactants, which of the following will occur ?
a)The activation energy would increase.
b)The speed of the reaction would decrease.
c)The number of effective collisions would increase.
d)The rate of the reaction decreases.
2.This graph represents the energy distribution of molecules at a given temperature.
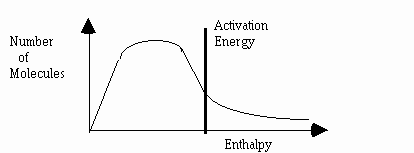
What will happen if a catalyst is added to the system ?
a)The shape of the curve will change.
b)The number of effective collisions will decrease.
c)The activation energy will shift to the right.
d)The activation energy will shift the left.
3.The following diagram represents the changes in enthalpy of a reaction.
Both the catalyzed and uncatalyzed pathways of the reaction are shown.
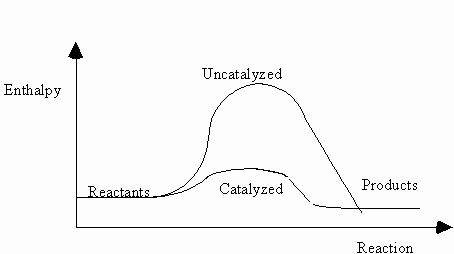
According to the diagram, which of the following statements is true ?
a)The activation energy of the catalyzed reaction is greater than that of the uncatalyzed reaction.
b)The heat of reaction of the catalyzed reaction is less than that of the uncatalyzed reaction.
c)The activated complex of the catalyzed reaction has less enthalpy than that of the uncatalyzed reaction.
d)The reactants and the products of the catalyzed reaction are different from those of the uncatalyzed reaction.
4. In the table below:
|
APPLICATIONS_ _ |
FACTORS |
|
a) refrigerating food to preserve it |
1. Nature of the substance |
|
b) cutting up a potato to cook it faster |
2. Pressure |
|
c) choosing platinum instead of other metals |
3. Surface area |
|
to reduce the rusting of electrical parts |
4. Temperature |
According to the factors which influence the speed of a reaction, which
of the following combinations correctly matches the factor with the application?
a)4a, 2b, 3c b) 2a, 1b, 3c c) 2a, 4b, 1c d) 4a, 3b, 1c
5.The conversion of sulfur dioxide SO2, to sulfur trioxide SO3, is represented by the following equation:
2 SO2(g) + O2(g) --> 2 SO3(g) + 206 kJ
Which of the following defines the rate of this reaction?
a)The number of moles of SO3 produced.
b)The number of moles of SO3 produced per unit time.
c)The mass of oxygen converted.
d)The quantity of energy released per mole of product.
6.You are supplied with three test tubes containing 0.10 mol/L of HCl at room temperature. A magnesium ribbon is immersed to different depths into the HCl.
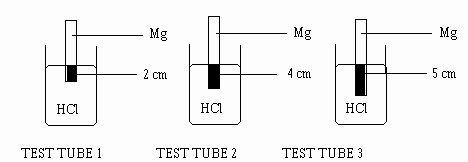
What effect will the length of immersed magnesium ribbon have on the rate or reaction ?
a)The rate of reaction in test tube 1 is 2.5 times faster than the rate of reaction in test tube 3.
b)The reactants being the same, there is no difference in the rate of reaction from one tube to the other.
c)The rate of reaction in test tube 2 is twice that of test tube 1.
d)Since the temperature is constant, the rate of reaction is the same in all three test tubes.
7.The formation of ammonia is represented by the following equation :
N2(g) + 3 H2(g) --> 2 NH 3(g) + energy
Which of the following best describes the rate of reaction ?
a)The volume of N2(g) involved in the reaction
b)The time required of 3 mol of H2(g) to be consumed
c)The time required for the energy of the reactants to be released
d)The number of moles of NH3(g) produced per unit time
8.SKIP When a chemical reaction is conducted at 25° C, it occurs in 60 s. The reaction is repeated at 10°C. How long might it take for this reaction to occur in 10° C ?
a) 6 s b) 24 s c) 60 s d) 150 s
9.List the following reactions in increasing order of speed of reaction.
All reactions at the same ambient temperature.
1. Pb+2(aq)
+ 2I-1(aq) --> PbI2(s)
2. 2 C2H2(g)
+ 5O2(g) --> 4CO2(g) + 2 H20(g)
3. Cu(s) + 4 HNO3(aq) -->2 NO2(g) + Cu(NO3)2
(aq) + 2 H2O(l)
a)1, 2, 3 b) 1, 3, 2 c) 3, 1, 2 d) 3, 2, 1
10. Which of the following factors always effect the rate of a chemical reaction ?
1. The nature of the reacting substances
2.The surface area of the reactants
3. The pressure
4.The concentration of the reactants
a)1, 2 and 3 b) 1, 2 and 4 c) 1, 3 and 4 d) 2, 3 and 4
11.When chalk reacts with an acid solution in a beaker, carbon dioxide is produced. Which factor DOES NOT AFFECT the rate of formation of carbon dioxide ?
a)The nature of the acid
b)The temperature of the acid
c)The size of the chalk
d)The concentration of the products of this reaction
PART B. Answer all questions on this sheet. Show all work. (3 marks each)
12.In the course of a
chemical reaction, the concentration of reactant A changed from 0.058 mol/L to
0.040 mol/L in 17 min. Find the reaction rate of this chemical reaction in mol/L/seconds.
13.As the saying goes " when a bottle of wine is opened, it becomes necessary to drink the wine". The reason for that is because the wine becomes oxidized with oxygen in the air. For each proper method, state the factor which will affect the speed of the reaction.
a)To put the bottle in the fridge without corking it.
b)To re-cork the bottle.
14.A reaction is represented by the following equation: 2AB --> 2A + B2
The graph below represents the number of moles of unreacted AB as a
function of the time since the beginning of the reaction.
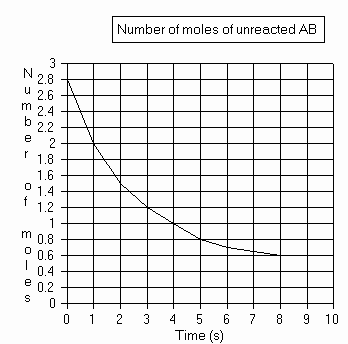
Complete the data table and draw a graph to illustrate the number of moles of A produced as a function of the time elapsed since the beginning of the reaction.
|
Time (s) |
Number of
Moles Unreacted AB |
Number of
Moles Reacted AB |
Number of
Moles A produced |
|
0 |
|
|
|
|
3 |
. |
|
|
|
5 |
|
|
|
|
8 |
|
|
|
Copyright ©2009
Created:April/6/1996; Updated:Dec/2/2009