Review for January Exam #4
PART A. Circle the correct answer.
1. Which of the following statements expresses BOYLE'S LAW(PV relationship) ?
a) At a constant pressure, the volume of the gas is directly
proportional to its temperature.
b) At a constant temperature, the volume of the gas is inversely
proportional to its pressure.
c) At a constant pressure, the volume of the gas is inversely
proportional to its temperature.
d) At a constant temperature, the volume of the gas is directly proportional
to its pressure.
2.Equal volumes of two different gases A and B are measured at the
same pressure and temperature. The masses of the two volumes are:
Gas A: 1.4 g
Gas B: 3.5 g
If Gas A has a molar mass of 28 g/mole, what is the molar mass of Gas B ?
a) 2.5 g/mole b) 11 g/mole c) 35 g/mole
d) 70 g/mole
3.A sample of oxygen gas occupies a volume of 600 mL.
If we triple the pressure of this gas while its temperature remains the same,
its new volume will be:
a) 200 mLb) 100 mLc)
1800 mLd) 300 mL
4.Consider the experimental set-up below. Case I is
given and by changing only the temperature and pressure, Case II and Case III
are achieved.
Case I contains 160 g of methane gas, CH4.
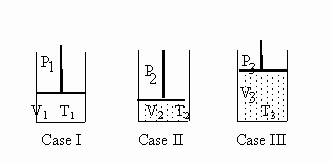
Which case, if any, contains the greatest number of molecules
?
a) Case Ib) Case IIc)
Case IIId) Each contains the
same number.
5.A gas is confined in a sealed cylinder. Which of the following is true
concerning the pressure of this gas when the absolute temperature is doubled ?
a) It is halved b) It is doubled c) It is tripled d) It remains the same
6.Sealed weather balloons are used to take certain meteorological
measurements. When the balloons rise in the atmosphere, their
volume changes. From the following factors, identify those which
influence
the balloon's change in volume.
1. Temperature 2. Type of gas 3. Pressure 4. Amount of gas in balloons
a) 1 and 2 b) 3 and 4 c) 1 and 3 d) 2 and 4
7.A container of a gas at 20°C is heated. What temperature must be reached
if the pressure of the balloon is doubled?
a) 40°C b) 273°C c) 313°C d) 400°C
8.When a gas is compressed with no change in temperature:
a)The spaces between the molecules become smaller.
b)Its molecules become smaller in size.
c)Its molecules occupy more space.
d)The molecules move slower.
PART B. Answer the following questions on the space provided. (3 marks each).
9.Find the new pressure of a gas originally at 105 kPa and occupying 500 mL, after
it is compressed to
100 mL while the temperature changes from 10 C
to 40 C.
10.
Given: 2 SO2(g) + O2(g)
--> 2 SO3(g)
a. If 3.5
moles of oxygen are consumed in the oxidation of sulfur
dioxide, how many litres of SO3 will be produced at 300 K and at a
pressure of 110 kPa?
b. If a 3.0 L tank at room temperature (20 C) is used to collect the sulfur trioxide that is produced from every gram of oxygen
consumed, to what pressure will the SO3 gas be subjected?
Copyright ©2009
Created:April/6/1996; Updated:Dec/2/2009