REVIEW 7 Chemistry
1. Which of the following represents the fastest rate?

A. 2.0 moles of NaOH disappearing in 400 s
B. 30 g of NaOH reacting in 1 minute
C. a reaction rate of 0.4 g NaOH/s
D. 0.10 L of 2.0 M NaOH
reacting in 10 seconds
Solution
Convert
each expression into moles/L.
A.
2.0 moles/400s = 0.005
moles/s
B.
30g/(40g/mole) = 0.75
moles
0.75 moles/60 s = 0.0125 moles/s
C.
(0.4g/40 g)/s = 0.01
moles/s
D.
n = CV = 2.0(0.10) =
0.20 moles
0.20/10
= 0.02 moles/s (fastest)
2.
A tin can is filled with steam until it reaches atmospheric pressure. Then,
after sealing the can, we continue to inject more steam into the can.

What will happen to the tin can? (5 points)
Answer Pressure
will increase because of the extra moles of gas within the can. The can could
eventually rupture. To get it to shrink we would have to cool the can to get
the steam to condense. This would lower pressure inside the can and allow
unhindered atmospheric pressure to crush the can.
 3.
3.
At a party that I hosted last year, the guests started to bore me with car-talk, so while I nodded my head, I thought of the following problem:
Assume 16 people just eating and talking for 4 hours. Each person burns an average of 297 kJ per hour eating and talking. Air has a specific heat of 1.0 J/(g.oC) and a density of 0.00129 g/cm3 = 1290 g/m3. The dining room has a volume of 3 m X 10 m X 6 m, and an area of 60 m2 through which it can lose heat to adjacent rooms. The dining area loses 69000 J/(hour* m2)
How much hotter will the dining room get?
Answer
Q = mcDT
The amount of heat absorbed by
the air in the dining room, Q, will be =
heat released by people – heat lost by the room’s walls =
16*297000 J /h ( 4
h) – 69 000 J/(h m2) * 4 h *60 m2 = 2 448 000 J
mass of air
= 1290 g/m3(3*10*6 m3) = 232 200 g
Q = mcDT
2 448 000 J = 232 200 g (1.0 J /(g C) DT
DT = 10.5 oC
4. The overall reaction for respiration is:
6 O2(g)
+ C6H12O6(s) + 38 ADP à
38 ATP + 6 CO2(g) + 6 H2O(l) (10 points)
How many litres of carbon dioxide would you exhale on
a cold day at –10OC and 105 kPa if your
body breaks down 480 g of glucose (C6H12O6(s))?
480 g /(180
g/mole) = 2.67 moles C6H12O6(s)
Ratio tells us that 6 * 2.67 = 16 moles of
CO2 will form.
V = nRT/P =
16(8.31)(-10+273)/105 = 333 L.
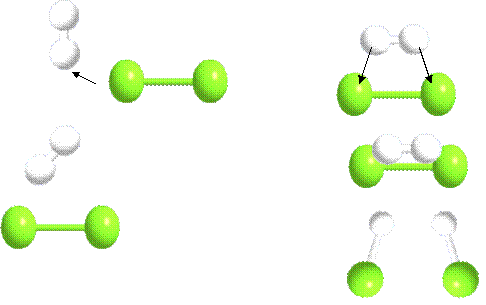
5. GOOD QUESTION ! With two drawings, show the difference between an ineffective and an effective collision between H2 and Cl2.
The product that could form is HCl.
Answer
|
INEFFECTIVE COLLISION |
EFFECTIVE COLLISION |
|
|
|
|
We have molecules hitting at the
wrong angle and with insufficient energy. No HCl
product is created from Cl2 and H2. |
Here
the collision is effective because the angle is right and the energy is
sufficient.(higher temperature) An activated complex
(in- between -product with Hmax)is
created . Note that with a higher energy you are more likely to get the right
angle just from the increased motion. Two molecules of HCl
are created. |
6. Which statement is FALSE as applied to the following reaction?
H2(g) àH(g) + H(g) DH = + 26 kJ
A) The positive DH means the reaction is endothermic.
B) Two moles of H gas contain more
energy than one mole of H2 gas.
C) Weight for weight, H gas would be
better fuel than H2 gas.
D) Heat is released when H gas is made from H2 gas.