Endothermic Versus Exothermic Reactions
To understand the difference between two types of reactions (exothermic and endothermic), we need to explore a couple of other concepts. In addition to kinetic energy (vibrational, rotational and translational motion), molecules also have potential energy. Potential energy is energy due to position and composition. It is stored in molecular bonds that exist within molecules (intramolecular); between different molecules (intermolecular), between different atoms of an element and finally within atoms.
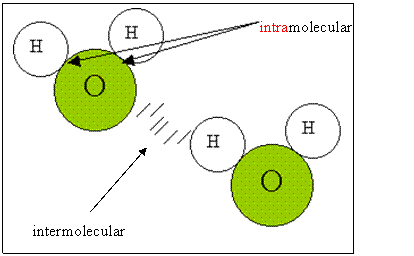 |
Example1 Show the intermolecular and intramolecular bonds in HCl.
H----Cl........H----Cl.......H----Cl
The intramolecular bonds are represented by -----, and the dots(......) represent the intermolecular bonds between the hydrogen od one molecule and the Cl of another HCl molecule.
Example 2 To make elemental sodium, one must first melt sodium chloride, and then using electrodes, electricity is forced through the molten compound so that electrons are forced back into sodium. It is a process that requires energy. How is this process related to the potential energy of sodium atoms?
Part of the energy used to create sodium from the electrolysis of sodium chloride is stored within the sodium atom as potential energy. When it was an ion and bonded to the oppositely charged chloride ion, it was at the bottom of the energy hill. With its loosely held valence electron and its potential to become strogly bonded to negatively charged ions, neutral sodium is a loose cannon, ready to fire!
The sum of all kinetic and potential energies of a substance is known as enthalpy (H). If in a reaction molecule A becomes molecules B and C, and if molecule A has more energy that both B and C combined, then the excess energy will be released into the environment. The environment becomes hotter; we have an exothermic reaction:
A à B + C + energy
Reactant products
On a graph exothermic reactions are represented as follows:
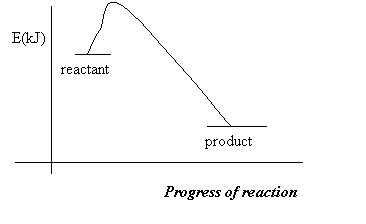 |
If we examine the graph more closely, we will notice that exothermic reactions have a negative change in enthalpy. A change in enthalpy, DH, is defined as the enthalpy of products – heat of reactants:
DH
= Hp - Hr 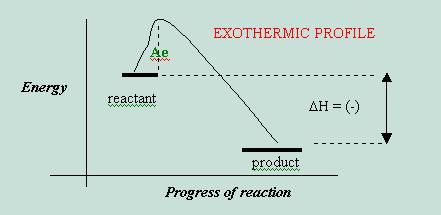
What is that little hill labeled, Ae? Ae = activation energy. This is the energy that reactants must absorb in order to form products, even if the products will not need the energy to store within their bonds. So Ae = Hmaximum - Hreactants
Examples of exothermic reactions:
- Digestion of food releases energy
- All combustion reactions (fires)
C + O2 à CO2 + energy
- Adding an alkali metal to water
2 Na + 2 H2O à 2 NaOH + H2 + energy
- Condensation of water
- Explosion of bombs
Endothermic Reactions
If substance A must take energy away from the environment in order to form product D, then the reaction is said to be endothermic, and the victimized environment will feel colder after the reaction.
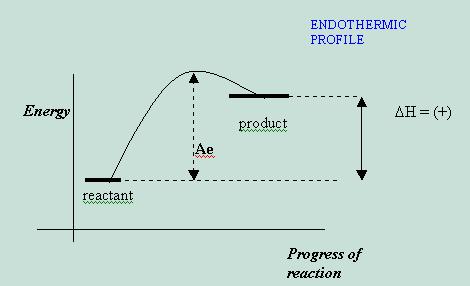 DH = (+) for endothermic
reactions and their profile looks like the following:
DH = (+) for endothermic
reactions and their profile looks like the following:
Examples of endothermic reactions:
- Melting of ice absorbs energy
- Dissolving ammonium nitrate in water( the essence of commercial cold packs)
NH4 NO3(s) + energyà NH4 NO3(aq)
- N2 + O2 + energyà NO