Solutions to Stoichiometry
1. For the reaction: 2K + 0.5 O2 à 1K2O
a. How many moles of O2 are needed to react with 0.56 moles of K?
0.56 moles of K 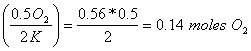
b. How many moles of O2 are needed to make 7.6 g of K2O?
7.6 g K2O (mole/94g K2O) = 0.0809 moles K2O
0.0809 moles K2O 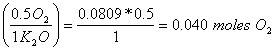
c. How many grams of K2O will be produced from 0.50 g of K?
0.50 g K (mole/39g K) = 0.0128 moles K
0.0128 moles K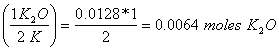
x = 0.0064 moles K2O (78+16)g/mole = 0.60 g
2. For the reaction: Na2O + H2O à2 NaOH
a. What mass NaOH could be made from 12.4 g of Na2O?
12.4 g of Na2O (mole/62 g) = 0.2 mole Na2O
0.2 mole Na2O 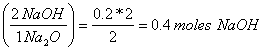
0.4 moles NaOH(40 g/mole) = 16 g NaOH
b. How many moles of Na2O are needed to make 1000 g of NaOH?
1000g (mole/40 g) = 25 moles NaOH
25 moles NaOH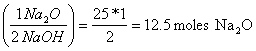
c. What would happen if 18 g of water were mixed with 18 g of sodium oxide?
18 g of H2O is 1 mole
18 g of Na2O = 18g(mole/62g) =0.29 moles
but according to the equation water and sodium oxide react in a 1:1, so we have too much water . All 0.29 moles of Na2O will react with only 0.29 moles of water to create twice as many moles of NaOH (see ratio). Answer = 0.58 moles of NaOH result.
0.58 moles(40 g/mole) = 23.2 g
3. Balance and answer the questions that follow:
C + 2 H2àCH4
a. How many moles of CH4 can be made from 7.0 g of H2?
7.0 g of H2= 3.5 moles H2. How? Divide by molar mass of diatomic hydrogen.
From the ratio, only half as many moles of methane will be produced so answer = 1.75 moles CH4.
b. What weight of H2 is needed to react with 5.0 g of C?
Convert 5.0 g of C to moles: 0.417 moles C
From ratio: 0.834 moles of H2 is needed.
Convert to mass by multiplying by molar mass:
1.66 g H2
c. What would happen if 20 g of hydrogen were mixed with 20 g of carbon?
20 g of C(mole/12 g) = 1.67 mole C. This amount needs 2(1.75 moles) = 3 moles of H2.(see ratio). We clearly have an excess of H2 since 20 g = 10 moles.
The amount of CH4 produced will depend on C, the limiting reagent, and only 1.67e moles of CH4 will be produced.
1.67 moles (16g/mole) = 27 g