Kinetic Molecular Theory
A. Main Points
- All gases consist of particles: either atoms or molecules. If the gas is monoatomic (like He, Ar), obviously it will consist of atoms. If it consists of I2, H2, N2, Br2, O2, Cl2, F2, then the gas will consist of molecules.
- The particles are in constant motion, and their volume is determined by their container, not by the size of individual molecules.
For a good animation of molecular motion, see the middle of the page at the following link.Type in "25" for number of particles, click "set" and then click "run".
- Their collisions are perfectly elastic, meaning that particles do not lose kinetic energy(energy of motion) by colliding with each other.
- For ideal gases only, the forces between particles are negligible. This is virtually the case for gases like helium, nitrogen and oxygen, but is not as valid for gases like H2O(water vapour) which have attractions for one another.
- Sometimes included as a point of the theory: the kinetic energy is directly proportional to the temperature of a gas in Kelvin degrees(absolute temperature). At a given temperature the molecules of all gases have the same average kinetic energy. That does not imply that they all move at the same speed. Lightweight ones move faster; heavier molecules are slower. But since kinetic energy depends on both mass and speed, it balances out: the avg. kinetic energy of a lightweight molecule will equal that of a slower heavyweight . Even if mass is the same, some molecules will move a little faster. But on average, at the same temperature, the speed of molecules will be the same for a given molecule of the same size.
B. The States of Matter
Solids Solids' particles vibrate. This is the only motion experienced by this state of
matter. Below is an example of the kind of vibrations experienced by water
molecules.
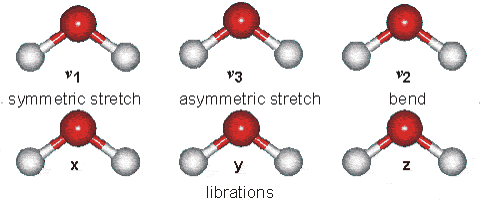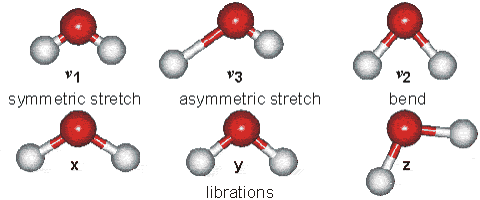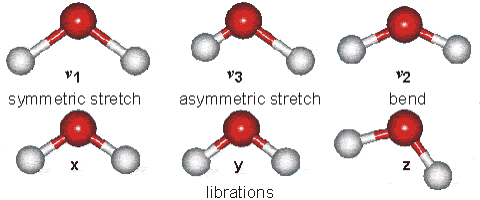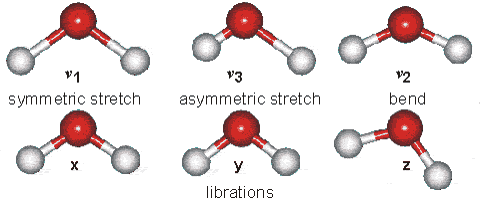
It is these vibrations that are responsible for the blue tinge of ice.

Liquids Liquid
molecules also vibrate but they rotate as well, giving them their familiar
freedom to assume the shape of whatever container they are poured in. When they
move they are constantly attracted to other molecues, making their motion quite
random. This is known as Brownian motion.
Gases Gas molecules
move from one point to another; they're said to translate. Of course gas
molecules still rotate and vibrate. Imagine the diver as a translating gas
molecule in the following image
C. Heat Versus Temperature
Heat is energy and it depends on temperature but also on the total mass of matter at that temperature. The temperature of a substance can only vary with the changing speed of its molecules. So a little 200 g cup of coffee at 60 C may have some pretty fast moving molecules but it will contain less heat than a cooler 30 C swimming pool containing 300 kg. Not convinced? Use Q=mc(Tf-Ti) from last year, using an initial temperature of 20 C for both.
D. Changes of State
What happens at the molecular level during changes of state(melting, evaporation, condensation etc)
Example 1: freezing
When water molecules are above zero, they vibrate and also rotate. As the temperature cools, the molecules begin to rotate more slowly. The attractions between molecules start to get stronger, and they begin to move into a geometrical pattern ( hexagon). At O C, many molecules stop rotating; the attractions are strong enough so that the molecules become locked into that pattern. Only vibrations exist. We have a solid; ice has formed from liquid water.
Example 2:evaporation
When molecules are in the liquid state, they vibrate and rotate. As the temperature rises, the vibrations and rotations become more intense. Eventually they absorb enough energy that the forces that keep the molecules together are overcome. They leave the surface of the liquid and begin to translate freely. At this point we have a gas.
D. Pressure
Pressure is force acting per unit area. Increasing pressure means that molecules will collide with both themselves and their container more frequently. So in chemistry pressure is also defined as "collision frequency".Note that in the diagram below, the arrows on each individual molecule, have the same total length, symbolizing that they are at the same temperature.
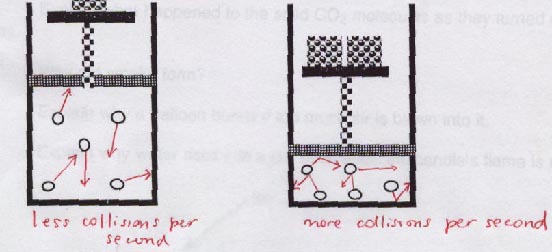
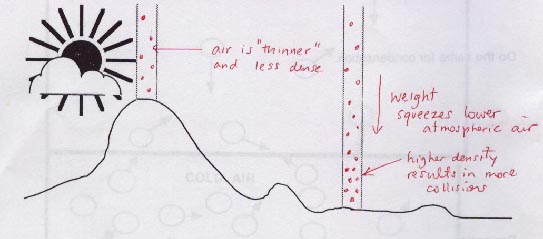
The second diagram to the right reveals why there is greater air pressure at sea level compared to higher altitudes.
The effect of atmospheric pressure is evident in everyday life. When the
plunger of a syringe is pulled up in a liquid, there is a low pressure inside
the syringe. This is because the liquid is in the way of the air trying to get
into syringe. Meanwhile the atmospheric pressure outside the liquid remains
just as strong, and the imbalance of pressure allows the liquid to be pushed
in, creating that familiar sucking effect. Of course, if you do not pull up on
the plunger, nothing happens because the atmospheric pressure is not strong
enough to both push down on the water and push up on the plunger.