Using Liquid Nitrogen to Demonstrate Charles' Law

Pics from LHA's open house. October 2004
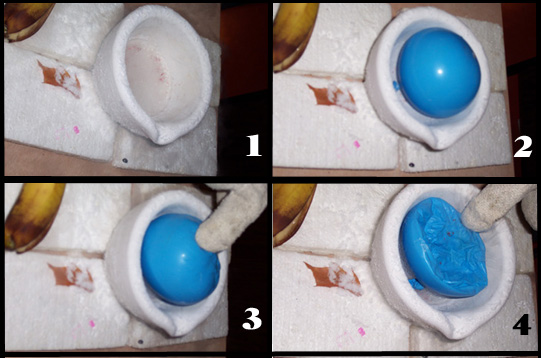
1 Liquid nitrogen was poured into a large mortar. (not the container of choice! We could not find anything else, but a thick styrofoam container would have served as a much better insulator).
2. Liquid nitrogen is at -196 °C or only 77 K. Compared to a room temperature of 20. oC = 293 K this represents only 77/293 of the absolute temperature so it should cause the volume of the balloon to be reduced to 77/293 or about 26% of its original volume.
3 The balloon is rotated to cool as much of its contents as possible.
4. As predicted, the air within the balloon now occupies a much smaller volume. If taken out of the liquid nitrogen, the balloon regains its original shape as its contents regain their lost kinetic energy.