Introduction
Morphine, C17H19NO3,
is the most abundant of opium’s 24 alkaloids, accounting for 9 to 14% of
opium-extract by mass. Named after the Roman god of dreams, Morpheus, who also
became the god of slumber, the drug morphine, appropriately enough, numbs pain,
alters mood and induces sleep. Less popular and less mentioned effects include
nausea, vomiting and decreased gastrointestinal motility. (It’s a great constipator, and in Guerin’s painting, Isis is perhaps
bringing Morpheus a laxative.) Morphine and its related synthetic derivatives,
known as opioids, are so far unbeatable at dulling chronic or so-called “slow”
pain, but unfortunately they are all physically addictive. During the American
Civil War, 400 000 soldiers became addicted to morphine.Both morphine and its hydrated form, C17H19NO3.H2O,
are sparingly soluble in water. In five litres of water, only one gram of the
hydrate will dissolve. For this reason, pharmaceutical companies produce
sulphate and hydrochloride salts of the drug, both of which are over 300 times
more water-soluble than its parent molecule. Whereas the pH of a saturated
morphine hydrate solution is 8.5, the salts are acidic. Since they derive from
a strong acid but weak base, they are both at about pH = 5; consequently,in maufacturing
stage,the morphine salts are mixed with small amounts
of NaOH to make them suitable for injection.
Morphine's Chemistry
The three dimensional structure of morphine is
fascinating. It consists of five rings, three of which are approximately in the
same plane. The other two rings, including the nitrogen one, are each at right
angles to the other trio.
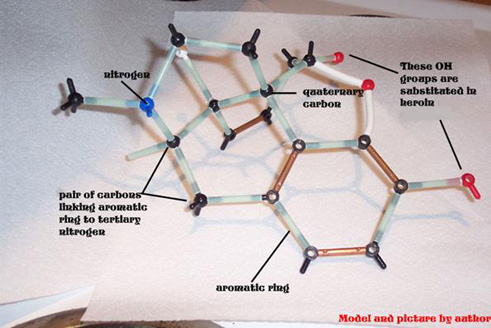
Opioid analgesics, including
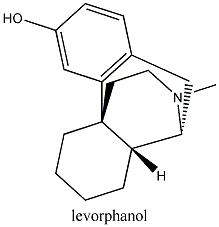
morphine, codeine, levorphanol, heroin and
structurally less similar drugs such as meperidine all have an aromatic ring
and a quaternary carbon atom linked to a tertiary amine group by two other
carbon atoms. This is known as the morphine rule,
but it should be pointed out that all of the opioids below also have a methyl
group attached to a nitrogen atom.
More
importantly and ironically, deviating from
the morphine rule has led to the creation of even stronger opioids such as fentanyl
and its ester-derivative, carfentynyl. These
molecules have tertiary carbons and in each case the atom is not linked to an
aromatic group.
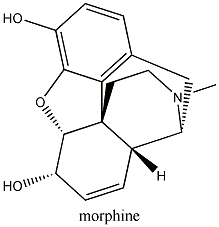
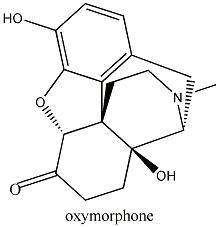
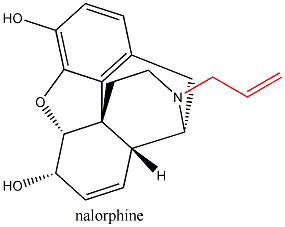
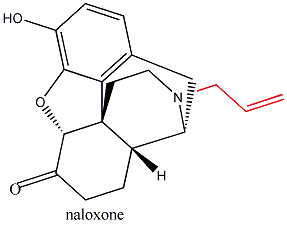
Substitute morphine’s methyl group with a propenyl
group, and you create nalorphine, an antagonist which counters morphine’s
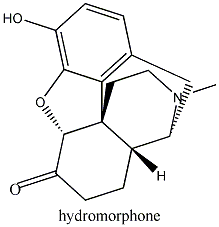
effects. Similarly, hydromorphone, a ketone version of morphine five times more
powerful than its parent molecule becomes the antagonist hydromorphone by the
same substitution of a CH3 with a CH2CH=CH2
group. More generally, morphine’s b-phenylethylamine
unit (essentially what I just described minus the quaternary carbon atom) is
also found at the tail-end of enkephalin molecules. Enkephalins are smaller versions of endorphins, which are
produced naturally in the brain, pituitary and other tissues, where they act
very much like opium’s molecules.
Agonists and Antagonists
Agonists
Morphine's Cousins
Antagonists
Physiology
Morphine acts on a specific receptor of nerve cells. More specifically
many such receptors are found in the spinal cord’s substantia gelatinosa, a region where pain signals are first
processed. The architecture of the morphine receptor is what dictates the
morphine rule. There is a flat part that binds to the aromatic ring, a cavity
that attracts the two carbon atoms and an anionic site that accommodates the
tertiary nitrogen atom. When morphine or another agonist binds to the receptor,
the cell membrane’s affinity for sodium ion changes. This eventually reduces
the release of neurotransmitters from the affected neurons. Investigators
learned about morphine’s mode of action by applying it and other opiates
(including enkephalin) to guinea-pig intestines.
(What else was gong to serve as the guinea pig for their experiments?) In the
presence of antagonists, Na+ affinity was restored and intestinal
contractions which had dropped precipitously shot up again.
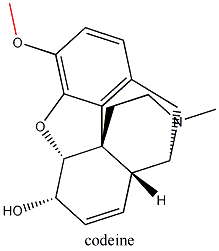
Not all opiates are created equally. Codeine is only 0.1 % as potent as
morphine in binding to receptors. But because codeine is converted to morphine
by the liver (all that has to happen is that the OCH3 group gets
replaced by OH) it becomes 20% as strong as the latter overall. In the late
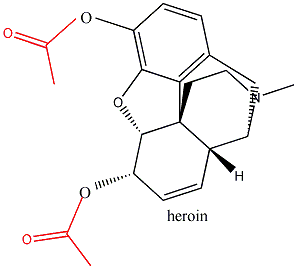
1800’s, the Bayer company, in hoping to come up with a non-addictive pain
killer, tried to acetylate both of morphine’s hydroxyl groups.
After all, this was in a way similar to how they had converted
salicylic acid into aspirin. But embarassingly, Bayer
invented heroin in the process.
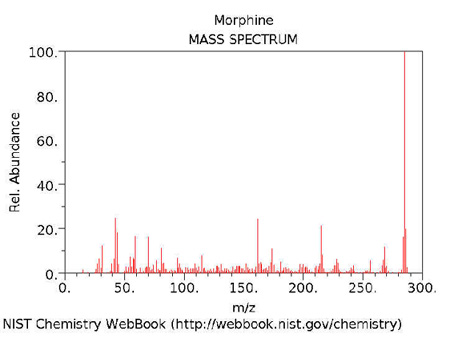
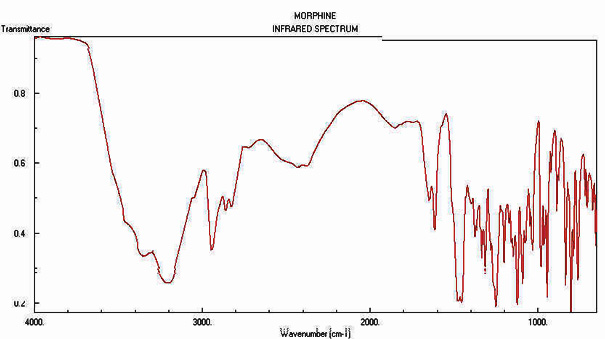
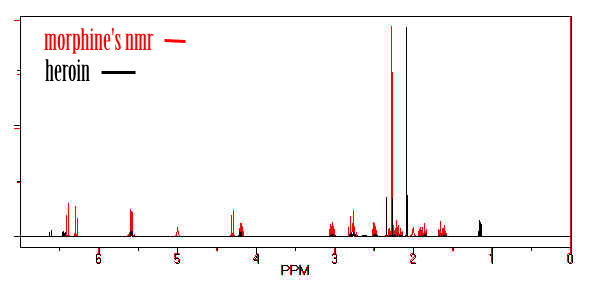
Analysis
The 1914 Harrison Narcotics Tax Act in the United States made possession of
morphine a criminal act. Forensic scientists exploit a group characteristic
property of morphine and its related compounds: a peculiar reaction with Mecke's (or Lafon's) reagent. The reagent consists of selenious acid (H2SeO3)in
concentrated sulfuric acid (H2SO4). If morphine and the
reagent react, the following colours appear: green,
then quickly a greenish blue, changing to blue, next
slowly to bluish green with a yellow-brown edge, then finally olivaceous green. This is one of a variety of color tests
that are used. Most, however, are presumptive tests, meaning that they suggest
the presence of opiates but don't absolutely prove it. Final confirmation is
obtained through instrumentation, either gas chromatography-mass-spectrometry
or by Fourier Transform Infrared Spectroscopy. NMR has also been used to identify heroin.