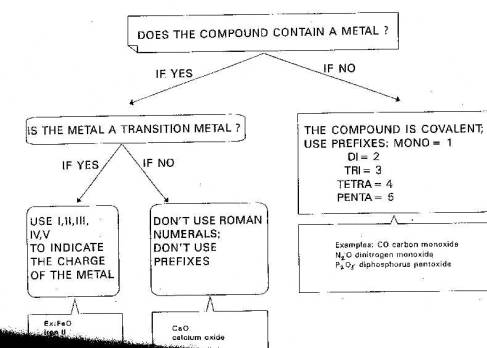
Naming Compounds
Before naming a compound, you have to figure out what kind of compound it is. We will consider three types:
- Ionic Compounds Without a Transition Metal.
Ionic compounds are formed when a metal gives up its electrons to a non-metal. Basically if the compound contains a metal, it is ionic. But there are different sets of rules for transition metals. A transition metal is an element with an atomic number of 21 to 30, 39 to 48 or 57 to 80.
a. So for a compound with any other metal, apply these rules:
- The metal ion's name does not change regardless of charge
- The non-metal's name ends in ide.
For example: AlCl3 = aluminum chloride
Na2S = sodium
sulfide
K2O = potassium oxide
MgH2 = magnesium hydride
Notice how the little numbers (subscripts) do not affect the way we name them.
b. In going backwards (from name to formula), we will have more fun. In such a case the total charge of the (+) and (-) ions in the compound has to be ZERO.
Example: What is the correct formula for calcium phosphide?
Here, we have to consider the common charges for calcium and phosphorus, which are +2 and -3, respectively. Calcium, if you recall, typically loses two electrons to get a noble gas electron arrangement, and phosphorus needs three more electrons.
Ca+2
and P-3
One of each would create a sum of 2 + (-3) = -1. To get a sum of zero, we need three Ca+2 ions and two P-3 for a total of 3(2)+ 2(-3) = 0.
So the answer is Ca3P2.
Other examples: potassium oxide : K+1 and O-2 give K2O
aluminum bromide Al+3 and Br-1 yield AlBr3.
c. Polyatomic Ions
When metals are bonded to polyatomic ions, which consist of two or more atoms with one overall charge, the same rules apply, but you have to learn the names and charges of common polyatomic ions.
|
Polyatomic Ion |
Name |
|
OH-1 |
hydroxide |
|
SO4-2 |
sulfate |
|
PO4-3 |
phosphate |
|
NO3-1 |
nitrate |
|
CO3-2 |
carbonate |
|
HCO3-1 |
hydrogen carbonate or bicarbonate |
|
ClO3-1 |
chlorate |
|
NH4+1 |
ammonium |
Na2CO3 = sodium carbonate. ( This is a useful chemical in purifying others; it is sometimes called washing soda)
KNO3 = potassium nitrate ( This is an ingredient of gunpowder and it is also found in fertilizer.)
To go backwards:
Aluminum
sulfate
This has Al+3 and SO4-2. To get a sum charge of ZERO, we need two aluminum ions and three sulfates, so the formula becomes Al2(SO4)3. Notice that when there is more than one polyatomic group, we make use of brackets.
2. Ionic Compounds With a Transition Metal.
The only difference here is that we have to specify the charge of the transition metal ion by using a Roman numeral, and keep in mind that a transition metal is an element with an atomic number of 21 to 30, 39 to 48 or 57 to 80.
|
Roman numeral |
Charge |
|
I |
+1 |
|
II |
+2 |
|
III |
+3 |
|
IV |
+4 |
|
V |
+5 |
|
VI |
+6 |
The reason we do this is not for the sake of nostalgia for bygone Roman numerals nor to imitate movie credits. Because transition metals can assume more than one charge, we have to specify which one is involved
Example: manganese(II) oxide contains Mn+2 and O-2. So we just need one of each and the formula becomes MnO.
Copper(I) oxide is Cu2O.
To go backwards, we need to figure out the charge of the transition metal.
Example: What is the correct name of CrCl3 ?
The charge of Cr is unknown = x . But chloride = (-1). The sum of the charges has top be zero, so:
x +3(-1) = 0.
x = 3.
Answer: CrCl3 = chromium (III) chloride.
3. Covalent Compounds. These are formed from non-metals that share electrons. Because there are many sharing possibilities between two non-metals, the formula cannot be guessed unless we have a naming system that reveals the number of atoms involved.
For this we use a set of prefixes:
|
Prefix |
Number of atoms |
|
mono |
1 |
|
di |
2 |
|
tri |
3 |
|
tetra |
4 |
|
penta |
5 |
|
hexa |
6 |
The only time we drop a prefix is if the mono is to appear at the beginning of the name.
Examples: CO = carbon monoxide ( note we don't say monocarbon monoxide)
CO2 = carbon dioxide
dinitrogen pentoxide = N2O5.
phosphorus trichloride PCl3.
Note that none of the above compounds contain a metal. Metals do not form covalent compounds, so we generally don't use prefixes for compounds containing metals.
Summary:
More Practice With Naming
1. Name the following:
a. NaOH b. Li2S c. FeCl3 (430 only) d. H2O e. K3N f. CaSO4
2. Write formulae for the following:
a. beryllium chloride b. copper (I) oxide (430 only) c. diphosphorus pentoxide d. aluminum carbonate e. ammonium phosphate f. sulfur dioxide
Answers
1. a. sodium hydroxide b. lithium sulfide c. iron(III)chloride d. dihydrogen monoxide e. potassium nitride f. calcium sulphate
2. a. BeCl2 b. Cu2O c. P2O5 d. Al2(CO3)3 e. (NH4)3PO4 f. SO2