Exercises
1. Hydrogen gas reacts with iodine according to the following equation:
H2(g) + I2(g) ŕ 2 HI (g)
a. From the point of view of HI, how would the rate of the reaction be expressed?
Dn/Dt, where n = moles of HI
b. How could you express the rate of the reaction from the point of view of hydrogen gas?
Dn/Dt, where n = moles of H2
c. If you had the necessary data, would you obtain the same number for both (a) and (b)? Why? Or why not?
No. Rate of HI production is 2 times greater because of molar ratio.
2. Experimental rate data was collected for the following reaction:
2 XY ŕ 2 X + Y2
The graph expresses the concentration of product X over the course of time.
Concentration
of Product X Versus Time
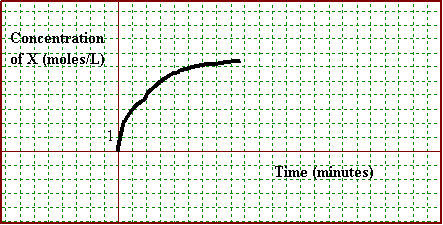
a. What was the average rate of formation of product Y2 in the first 8 minutes? Express in moles per liter per minute and in moles per liter per second.
For X, Dn/Dt = (6.2 – 0)/(8-0) = 0.775M of X /min
But 1Y2/2X(0.775M of X /min) = 0.3875 M of Y2/min
b. At what rate was XY decomposing between the 5th and 10th minute?
From ratio, rate of X = that of XY
Dn/Dt = (6.5 – 5.9)/(10-5)= 0.12 M XY/min
d. At what rate was Y2 forming between the 5th and 10th minute?
1Y2/2XY(0.12 M of XY /min) = 0.060 M of Y2/min
d. Use the graph to complete the following table. Assume a 1.0 L flask.
|
Time |
Amount of XY
that reacted (moles)(same
as X that appeared;see ratio) |
Amount of XY
remaining (moles) |
Amount of Y2
forming(moles) (half of X that appeared;see
ratio) |
|
0 |
0 |
12 |
0 |
|
1 |
3 |
12-3 = 9 |
1.5 |
|
2 |
4 |
8 |
2 |
|
3 |
5 |
7 |
2.5 |
|
4 |
5.6 |
6.4 |
2.8 |
|
5 |
5.9 |
6.1 |
2.85 |
|
6 |
6.1 |
5.9 |
3.05 |
|
7 |
6.2 |
5.8 |
3.1 |
|
8 |
6.3 |
5.7 |
3.15 |
e. Using the above table, graph the concentration of remaining XY versus time.
3. Consider the reaction: N2(g) + 3 H2(g) ŕ 2 NH3(g)
Under a certain set of conditions, the rate of formation of ammonia was found to be 24 L/minute. At what rate was hydrogen being consumed?
(3/2)( 24 L/minute)=36 L/minute. Because of Avogadro’s hypothesis, moles of gas and litres are proportional as long as conditions are the same.
4. a. The electrolysis of water produces hydrogen gas according to the following equation:
2 H2O(l) ŕ 2 H2(g) + O2(g)
A chemist wants 24.0 g of oxygen using an apparatus that decomposes 45.0 mL of water per hour at room temperature and pressure. How many minutes will it take to make that much gas?
45.0 ml H2O/h = 45.0 g H2O /h
(mole/18 g) = 2.5 moles H2O /h
2.5 moles H2O /h [ 1 mole of O2/2
molesH2O] = 1.25 moles O2/h
= 1.25 moles O2/h (32 g/mole) = 40 g O2/h
24.0 g/(40 g O2/h) = 0.60 h (60 min/h) = 36 minutes.
b. If under a different set of conditions it took an hour to make 200 g of oxygen gas, at what rate was the water decomposing in moles/minute?
200g O2/h
(mole/32 g)(1h/60 min) = 0.104 moles O2
/minute
water decomposed at 0.208 moles /minute or 3.75 ml/minute or 225 ml/hour