Solutions to p48
1.†††††††† a.†††††††† fast:††††† this is a reaction in which aqueous ions produce a precipitate.
b.†††††††† fast:††††† neutralization reaction
c. slow: covalent bonds are being broken
d.†††††††† slow: covalent bonds are being broken, but probably not as slow as c because less bonds have to be broken)
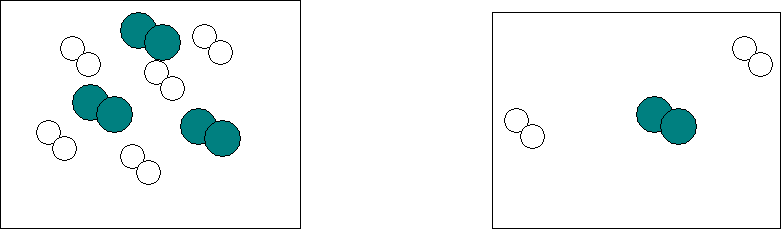
2.†††††††† Higher concentration( on the left) increases the likelihood that hydrogen(white) will collide with iodine(blue). There is more crowding than there is on the right.
3.†††††††† 2 kg of sliced potatoes will cook faster because of their higher surface to volume ratio.
4.†††††††† Each quarter piece will now have an area of †(4pr2)/4 + pr2 = 2 pr2. The total surface area of the four pieces will be 8 pr2. The uncut Zn had a surface area of †4 pr2, so the cut zinc will react 8 pr2/ 4 pr2 = twice as fast. More details.
5.†††††††† The activation energy will be lowered as shown below:
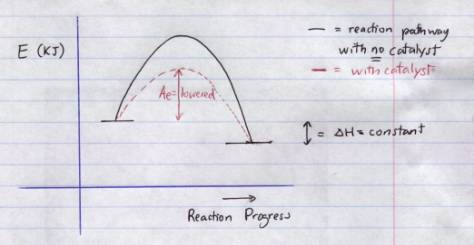
6.†††††††† With an inhibitor activation energy will increase for both endo and exo reactions.
7.†††††††† Cl reacts with O3 to create ClO and O2, and eventually the ClO returns the Cl to the reaction cycle by reacting with O (monoatomic oxygen), recycling the Cl and making it available again for destruction of more ozone.
8.†††††††† The penicillin molecule has a similar shape to one of the molecules that bacterial enzymes use to build cell walls. But when penicillin binds to that key site on the enzyme, the bacteria cannot synthesize the exact molecule needed for its wall. Without new cell walls bacteria canot reproduce or make repairs to their existing cells. They die.