 The Chemistry of Pool Water
The Chemistry of Pool Water
One of the first things I noticed when I bought a pool is that pool chemistry-terms are confusing to both lay people and specialists alike. A pool manual’s usage of terms such as “alkalinity” and “free chlorine” are not textbook definitions. But once I sorted through the initial confusion, it gave me one more excuse to do some simple experiments in my backyard.
1. Chlorine and pH
When pool manufacturers use the term “free chlorine”, they are referring to hypochlorous acid, HOCl, which exists in aqueous solution. This is the chemical species that actually kills bacteria and attenuates algal growth, preventing diarrhea, swimmer's ear (a nasty earache) and various skin and respiratory problems. Cl2, diatomic chlorine, would also do the trick, but it is far too dangerous.
If Ca(ClO)2 is used as an alternative, the product will slowly dissolve in water to produce the hypochlorite ion, OCl-(aq).
But OCl-(aq) goes into equilibrium with the vital HOCl.
OCl-(aq) + H2O = HOCl (aq) +
If the pH is too high, the reverse reaction will
be encouraged, leading to a higher concentration of OCl- but lowering the amount of essential HOCl. Although a lower pH(more
acid) will discourage the forward reaction (by destroying
The ideal concentration of hypochlorite in pool water is about 2 parts per million, translating into about 2 mg of calcium hypochlorite per liter of water. I normally start with a little more, especially if I’m not chlorinating at dusk (best time). Ultraviolet light will break down chlorine compounds, create Cl free radicals and eventually convert them to HCl. If I dissolve 120 g in a 40 000 L pool, that’s equivalent to 3 g per 1000L, which is 0.003 g/L or 3 mg per L.
On average, such an amount will last two days.
But factors such as temperature, frequency of use, presence of a pool cover
(which filters UV) will either increase or decrease HOCl’s
residence-time. There are times in the summer when my HOCl
runs low after 24 hours. Conversely, in the spring before we actually start
swimming in the pool, I have seen chlorine levels remain adequate beyond 48
hours.
 Adding
more than the required amount of hypochlorite is not recommended because it is
an eye and skin irritant.
Adding
more than the required amount of hypochlorite is not recommended because it is
an eye and skin irritant.
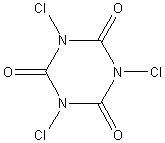
For those seeking a more stable chlorine-product and the illusion of convenience, trichlor (trichloroisocyanuric acid) tablets (or “pucks”) are available.
In addition to generating HOCl, trichloroisocyanuric
acid lowers pH and drives the HOCl-equilibrium to the
right (good old LeChatelier principle), but unless
the owner periodically adds carbonate ion (so-called pH+), the acid will
accumulate and take its toll on pool equipment. It is for this reason that I
prefer Ca(ClO)2.
Although it too has its drawbacks (calcium deposits can accumulate if dilution
and backwashes are not routinely performed) it is nowhere near as nasty as trichlor. Thanks to trichlor and some carelessness
on my part, a couple of years ago I almost became a 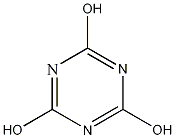 World
War I-like victim of chlorine gas. Here’s how.
World
War I-like victim of chlorine gas. Here’s how.
 First
trichlor creates HOCl and isocyanuric acid:
First
trichlor creates HOCl and isocyanuric acid:
+ 3 H2O =
3
HOCl +
With both acid and HOCl around, elemental chlorine can potentially be part of the following equilibrium:
HOCl(aq) + HCl (aq) = H2O(l)
+ Cl2![]()
Normally the acid introduced by the pucks, once diluted
by a pool’s large volume of water, will not create much of the elemental
chlorine gas. But, I had a leak in my storage shed, and somehow water crept
past what I thought was a waterproof lid. The water in the bucket of trichloroisocyanuric acid pucks partially dissolved the
chemical, creating very high concentrations of both HOCl
and HCl. So when I opened the container I was greeted
by the second-most vicious member of the halogen family. The greenish gas felt
like a knife going through my throat and lungs. Luckily I was alone and
outdoors. Good ventilation and a strong coughing relex
flushed most of it out of my system.
2. Alkalinity
Alkalinity to a chemist means base-level. A base is an acid’s counterpart. But to a pool person, alkalinity refers to a specific base, namely hydrogen carbonate ion (HCO3-). The reason it’s important to have a decent amount of this in your pool, is that it acts as a buffer, thus stabilizing the pH level.
3. Measuring pH
The typical pH paper provided in some kits is not at all
accurate. It can easily be off by more than 1 unit, and a single unit on the pH
scale, a logarithmic one, is really a factor of 10 in terms of H+.
Some kits come with phenol red, but it is only useful between pH’s of 7 and 8. A pH meter would be nice, or you can embark
upon the following adventure.
If the water is
more acidic than 7---tap water and rainwater typically are----you
will first need bromothymol blue. Take a small sample
of tap water and one of pool water. Since the indicator bromothymol
blue is dissolved in NaOH, adding four drops to a
small vial containing an unbuffered sample will not
give the expected green colour for tap water. It will
be blue. If your pool water plus four drops of the same indicator is a lighter
blue, or greenish blue, it will obviously be more acidic than tap water. It’s
time to add carbonate in proportion to the volume of the pool, and it will
correspond to a pH of <6.8 in the chart in the back of the pH+ bottle. Then
after 12 hours, remeasure the pH using phenol red
indicator. If it’s still below pH = 7.2, add more carbonate. If you
accidentally overshoot the desired value, it will be time to buy pH(-), and you’re on your way to having your own lab.
In fact pH(-), which contains NaHSO4, is necessary for those who use Ca(ClO)2. The latter is slightly alkaline when pure, since it is derived from a strong base and a weak acid, but when calcium hypochlorite reacts with carbon dioxide from the air, chlorine and alkaline calcium carbonate are produced. So after about month of chlorinating the ph climbs to about 7.8 which is too high for a sufficient amount of HOCl to remain in the water.
4. Chlorine Indicator
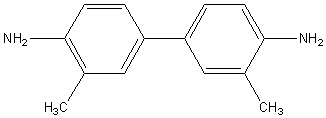 A
common agent used to measure free chlorine is orthotolidine,
which turns either a light or dark shade of yellow depending on the
concentration of HOCl. Ortho-tolidine
is unfortunately a cancer-suspect agent, so I wear gloves, and I do not dump
the samples back into the pool. Interestingly, the
A
common agent used to measure free chlorine is orthotolidine,
which turns either a light or dark shade of yellow depending on the
concentration of HOCl. Ortho-tolidine
is unfortunately a cancer-suspect agent, so I wear gloves, and I do not dump
the samples back into the pool. Interestingly, the
Since it’s vital to constantly monitor pH and chlorine levels in one’s swimming pool--too much chlorine is toxic; too little leads to problems; too little acid will not let HOCl do its job, and too much will be corrosive--- a chlorine indicator is indispensable. Hopefully, a safer one will soon be commercially available. Otherwise, I will have to rely on the old iodide-thiosulfate titration.
References:
Selinger, Ben. Chemistry in the Marketplace. Allen & Unwin Academic. 1998
Tamminen, Terry. The
Ultimate Pool Maintenance Manual McGraw-Hill. 2000
http://www.rhtubs.com/chlorine.htm#trichlor
http://en.wikipedia.org/wiki/Calcium_hypochlorite
http://www.chemicalland21.com/specialtychem/finechem/o,o'-TOLIDINE.htm
http://www.freepatentsonline.com/5223617.html
http://www.edstrom.com/DocLib/MI4148.pdf
Page Maintained by Enrico Uva
Technical Asistance: C. Frizzell; and M.Pololos, D.Verrillo and K.Papoulias at
EMSB
Comments: euva@retired.ca
or
euva@prof.emsb.qc.ca
The
best things in life are free: the chemistry of love and the love of chemistry.
Fleeting thought:
The product of the electron’s mass and radius is related to that of the
neutron.
(see rigdenextra.html)
.
Copyright ©2007
Created:January/1/2001;