The Chemistry of Swimming Pools
One of the first
things I noticed when I bought a pool is that pool chemistry-terms are
confusing to both the lay person and to a person with a chemistry background.
There is a lot of jargon, which means nothing to a non-chemist, and terms like
“alkalinity” and “free chlorine” can be easily misinterpreted by someone who
knows their textbook definitions.
1. Chlorine and pH
When pool
manufacturers use the term “free chlorine”, what they are actually referring to
is the chemical hypochlorous acid, HClO, which exists in aqueous solution. This is the actual
substance that kills bacteria. By doing so, HClO
prevents diarrhea, swimmer's ear (a nasty earache) and various respiratory, skin
and infections of minor cuts. Cl2,
diatomic chlorine, would also do the trick, but it is more dangerous.
So when you add, say,
Ca(ClO)2 to your
pool, the product will slowly dissolve in water to produce OCl-(aq).
But this ion creates an equilibrium with water, in other words a pair of
reactions that occur at the same rate:
ClO- + H2O = HOCl +
If the pH is too high
the reverse reaction will be encouraged leading to a higher concentration of ClO- , but lowering the amount of essential HOCl. Although a lower pH(more
acid) will discourage the forward reaction (by destroying
Of course, although a
bit of chlorine kills bacteria, too much would irritate eyes, lungs and so
forth. After all it was used as a chemical weapon in
On average such an
amount will last two days. But factors such as temperature, use, presence of
pool cover(which filters UV) will either increase or
decrease HOCl’s residence-time.
This is important
because trichlor tablets are sold with high levels of
cyanuric acid, which is included to drive the HOCl-equilibrium to the right, but unless the owner
constantly adds carbonate ion(so-called pH+), the acid will take its toll on
pool equipment. For this reason I prefer Ca(ClO)2, but it too has its side effects, as
calcium deposits can accumulate, if dilution and backwashes are not routinely
performed.
So this is why it is
important to constantly monitor pH and chlorine levels in one’s swimming pool.
Too much chlorine is toxic; too little leads to problems. Too little acid (high
pH) will not let HOCl do its job, and too much will
be corrosive.
2. Alkalinity
Alkalinity to a chemist means base-level. A base is an acid’s
counterpart. But to a poolster, alkalinity refers to
a specific base, namely hydrogen carbonate ion (HCO3-).
The reason it’s important to have a decent amount of this in your pool, is that
it acts as a buffer and stabilizes the pH level.
3. Measuring pH
The typical pH paper provided in some kits is not at all accurate. It
can easily be off by more than 1 unit, and a single unit on the pH scale, a
logarithmic one, is really a factor of 10 in terms of H+. Some kits come
with phenol red, but it is only useful between pH’s of
7 and 8. If the water is more acidic than 7---tap water and rainwater typically
are----you will first need bromothymol
blue. Take a small sample of tap water and one of pool water. Since the indicator
bromothymol blue is dissolved in NaOH,
adding four drops to a small vial containing an unbuffered
sample will not give the expected green colour for
tap water. It will be blue. If your pool water plus four drops of the same
indicator is a lighter blue, or greenish blue, it will obviously be more acidic
than tap water. It’s time add carbonate in proportion to the volume of the
pool, and it will correspond to a pH of <6.8 in the chart in the back of the
pH+ bottle. The after 12 hours remeasure the pH using
phenol red indicator. If it’s stillbelow pH= 7.2 add
more carbonate. If you accidentally overshot the desired value, it will be time
to buy pH(-), and you are on your way to having your
own lab.
4. Measuring Chlorine
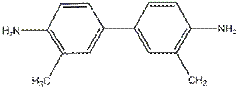
A common agent used
to measure free chlorine is ortho-tolidine, which
turns either a light or dark shade of yellow depending on the concentration of HOCl. Ortho-tolidine is
unfortunately a cancer suspect agent.
References:
http://www.rhtubs.com/chlorine.htm#trichlor
http://en.wikipedia.org/wiki/Calcium_hypochlorite
http://www.chemicalland21.com/specialtychem/finechem/o,o'-TOLIDINE.htm