Key Concept: Periodic Trends (436 only)
Sample Questions:
1.(JANUARY 2000 430): The atomic size of an element is an example of a periodic property. Which of the following concerning size and periodicity are TRUE?
- Within a family, atoms get smaller with increasing atomic number.
- Within a period, atoms get smaller with increasing atomic number.
- Within a family, atoms get larger with increasing atomic number.
- Within a period, atoms get larger with increasing atomic number.
- 1 and 2
- 1 and 4
- 2 and 3
- 3 and 4
2.(JUNE 1995 436): Which of the following describes how atomic masses vary with atomic number?
- As atomic number increases, atomic masses decrease with regularity.
- As atomic number increases, atomic masses increase and decrease in a cyclical fashion.
- With a few exceptions, atomic masses increase with increasing atomic number.
- With dozens of exceptions, atomic masses increase with increasing atomic number.
3. (AUGUST 1994: 416) The following graph shows how electronegativity varies
with atomic number.
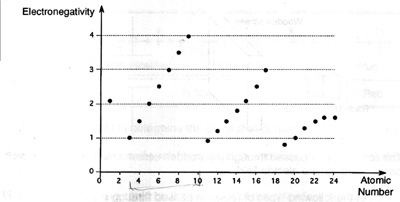
According to this graph, which of the folllowing statements is TRUE ?
- In period 2, electronegativity increases with increasing atomic number.
- In period 2, electronegativity decreases with increasing atomic number.
- In period 2, electronegativity does not change with increasing atomic number.
- In period 2, electronegativity increases and then decreases with increasing atomic number.
Notes:
Electronegativity
This is a measure of an atom's tendency to pull electrons
towards itself while bonded to another atom. In a sense, it is a measure of
greediness. The nonmetals, which are close to having a full energy level, are
far more electronegative than metals.
Within any period, as atomic number increases, electronegativity increases.
For the nonmetals, within a family, electronegativity decreases
with increasing number. So fluorine, for instance, is the most electronegative
halogen; in fact it is the periodic table's most electronegative atom.
Atomic Volume or Radius
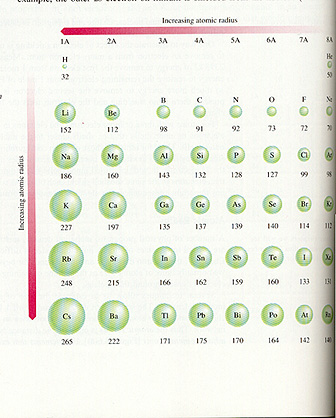
The above illustration reveals how, within a family, not surprisingly,
atomic volume increases with increasing atomic number. But note that across a
period (from left to right), atomic volume actually decreases. This is because
additional nuclear charge is acting on the same number of shells, so the extra
charge squeezes the shells more tightly.
Melting Point and Boiling Points
For alkali metals, both melting points and boiling points decrease with
increasing atomic number. So Fr is the lowest-melting alkali metal.