Chemical Formulas
Formulas show the makeup of a compound: how many atoms of each type are bonded together. When atoms bond together, regardless of whether they are those of elements or compounds, we call them molecules
· For water:
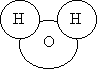
H2O =
Notice that the number applies to the symbol to the left of the number. We have drawn one molecule of water.
· For carbon dioxide

 CO2
=
CO2
=
Notice that the "2" does not apply to the carbon.
More examples:
|
Formula |
Number of Atoms in each Molecule |
|
BeCO3 |
Be: 1 C: 1 O: 3 |
|
Al(NO3)3 |
Al : 1 N: 1 X 3 = 3 O: 3 X 3 = 9 |
|
(NH4)2S |
N: 2 H: 4 X 2 = 8 S: 1 |
|
|
|
Also notice that for in a single molecule of ammonium sulfide, [(NH4)2S], there are 11 atoms in all, of three different types.