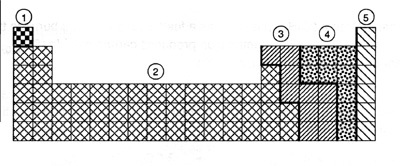
Key Concept: Metals, Metalloids and Non-Metals
Sample Questions:
1.(JANUARY 1995 416): After performing tests on several elements, you note these properties:
1.They are ductile and malleable;
2. They are good conductors of electricity;
3. They react with acids.
In which region of the periodic table are the elements with these properties located?
2.(JUNE1999 416): In the lab you are given a solid and told that it is a metalloid.
In order to verify this,
| Grouping | Examples | Location | Physical Properties |
|---|---|---|---|
| Metals | Alkali metals, alkaline earth metals, transition metals(Sc, Ti, V etc) | With exception of H and metalloids, all elements to the LEFT of staircase. | good conductors of electricity and heat; shiny, malleable, usually high density and high melting ,except for alkalis;many react with acid |
| Metalloids | B, Si, Ge, As, Sb, Te, Po: | With exception of Al, elements that border the staircase (jagged line in periodic table) | semi-conductors; some shiny; don't react with acid; not malleable |
| Nonmetals | N, O, S, P, Cl, Br, Se etc | With exception of metalloids and noble gases, elements to the right of the staircase. | poor conductors of heat and electricity; low-melting |