1.a.
CH4
+ 2 O2
![]() CO2
+ 2 H2O
CO2
+ 2 H2O
b.
2 Cu
+ O2
![]() 2 CuO
2 CuO
c. 2
Be
+ O2
![]() 2 BeO
2 BeO
d. Fe(NO3)2
+ 2 NaOH
![]() Fe(OH)2
+ 2 NaNO3
Fe(OH)2
+ 2 NaNO3
e.
2 NaBr
+ CuCl2
![]() 2 NaCl
+ CuBr2
2 NaCl
+ CuBr2
f.
Al2(SO4)3
+ 3 Ca(OH)2
![]() 2Al(OH)3
+ 3 CaSO4
2Al(OH)3
+ 3 CaSO4
g. Pb(NO3)2
+ 2
KI ![]() 2 KNO3
+ PbI2
2 KNO3
+ PbI2
h. CaCl2
+ BaF2
![]() CaF2
+ BaCl2
balanced
CaF2
+ BaCl2
balanced
i.
4Na
+ O2
![]() 2Na2O
2Na2O
j.
2 Li
+ 2 H2O ![]() 2 LiOH
+ H2
2 LiOH
+ H2
k.
2 PbCrO4 + 16 HCl + 6 FeSO4
![]() 2 PbCl2
+ Cr2(SO4)3
+ 4 FeCl3 + 8 H2O + Fe2(SO4)3
2 PbCl2
+ Cr2(SO4)3
+ 4 FeCl3 + 8 H2O + Fe2(SO4)3
l.
2 NO + O2
![]() 2 NO2
2 NO2
m.
2 C
+ H2
![]() C2H2
C2H2
n.
AlCl3
+
4NaOH ![]() NaAlO2 +
3 NaCl
+ 2 H2O
NaAlO2 +
3 NaCl
+ 2 H2O
o. 4 FeO +
O2 ![]() 2 Fe2O3
2 Fe2O3
2. Draw 1b and 1j after they
have been balanced.
Li
![]() j
j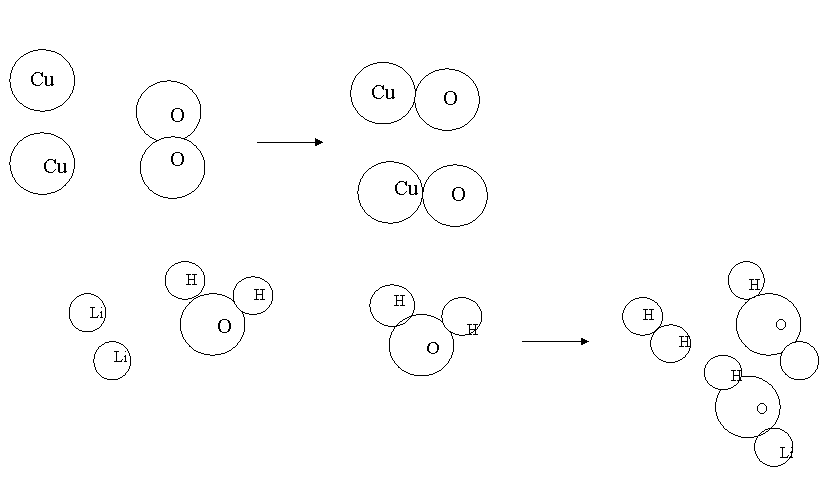
3. Translate 1i and 1j into words.
i) 4Na
+ O2
![]() 2Na2O
2Na2O
4 parts of sodium react with one part of oxygen
to produce two parts of sodium oxide
2
Li
+ 2 H2O ![]() 2 LiOH
+ H2
2 LiOH
+ H2
2 parts of lithium react with 2 parts of water
to produce 2 parts of lithium hydroxide and 1 part of hydrogen gas