1.
|
Homogeneous
Mixture |
Solvent |
Solute(s) |
|
a. 18 K gold (24 K is pure) |
Gold |
Copper,
silver etc |
|
b. Air |
Nitrogen |
Oxygen,
argon, CO2, H2O |
|
c. ocean water |
water |
Na+,
Cl-, HCO3-1, Mg+2, Br-1 |
|
d. 7up |
water |
Sugar,
CO2, flavours |
2. Why
does salt seem to disappear as it dissolves in water?
Water breaks up the crystal and
separates the ions.
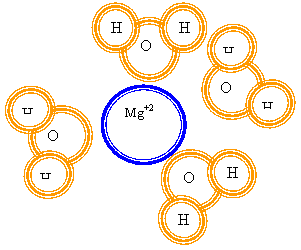
3. What
part of the water molecule faces a dissolved Mg+2 ion? Draw it.
The oxygen faces it
4. Complete
and balance the following ionic equations:
a. KBr(s) à
K+1(aq) + Br-1(aq)
b. CaCl2(s) à
Ca+2(aq) +
2 Cl-1(aq)
c. (NH4)2S(s)
à
2NH4+1(aq) + S-2(aq)
d. Na2CO3(s) à
2 Na+1(aq)
+ CO3-2(aq)
e. AlPO4(s) à
Al+3(aq) + PO4-3(aq)
f. AlCl3(s) à
Al+3(aq) + 3 Cl-1(aq)
g. AgNO3(s)
à
Ag+1(aq) + NO3-1(aq)
h. ZnCl2(s) à
Zn+2(aq) +
2 Cl-1(aq)
i. KOH(s) à
K+1(aq) + OH-1(aq)
j. HF(s) à
H+1(aq) +
F-1(aq)
k. RbBr(s) à
Rb+1(aq) + Br-1(aq)
l. Ca(NO3)2(s)à
Ca+2(aq) +
NO3-1(aq)
m. Al2S3(s) à
Al+3(aq) +
S-2(aq)
4. If 4.0 grams of Na3N dissolved in
water, what mass of ions would be created in solution?
Answer: 4.0 grams ( mass is
conserved!)
5. (430 only) How many moles of sodium
would appear in solution for every gram of sodium nitride (Na3N)
that dissolved?
Na3N(s)
à 3
Na+1(aq) + N-3(aq) Yeap! That’s right. We need to write a
balanced equation before ink and mistakes leak out of our pens.
1.0 g(mole/83g/mole)
= 0.0120 moles of Na3N(s)
Examine ratio:
there will be 3(0.0120) = 0.036 moles of Na+1 in solution