Solutions to Phys Sc June 1996
- C
- B
- D
- C
- D
- A
- D
- C ( high resistance; low conductivity)
- D ( liquid water turns the turbines)
- C
- B
- A (G=I/V)
- C
- C [V= IR= 0.5(10+20)]
- D
- A
- D [ 2/0.100L]
- C
- B
- C
- C
- D [0.20(29+34)+0.80(29+36)
- A [Think of Al+3 and O-2]
- B [ 1A = 1C/s; also note that I=Q/t is given to you as a formula on exam]
- B
- D[E=VIt=120(120/10)(3*3600s/h) J=1552kJ.
- B [ V=IRe = 2(8)=16 V]
- D [ rest have no metal]
- B [2(+3) + 3x = 0; so x = -2.]
- C [0.250*0.5*(24+28+96)]
- A) Measure 10 ml of the liquid precisely using a pipette.
B) After taring another container on a balance, pour the liquid into the container.
C) Find the mass.
D) density= mass/volume
32.
- Since A and B attract, Bís charge is opposite that of A. B = (+)
- Since B and C attract, Cís charge is opposite that of B. C = (-)
33.
- O
- Ar
- B
- Na
- Ca
34.
V= I2R2 =5(20) = 100 V
100=I1R1=I1(10)
I1 =10 A
IT= 10 + 5 = 15 A
35.
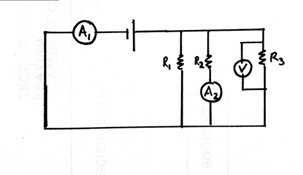
36.
Cost = Energy* rate = Power*time*rate=1.5kW(330h)($0.05/kWh) = $24.75
37.
C1V1 = C2V2
48(0.100) = C2(0.100 + 0.200)
C2= 16 g/L
38.
- Begin with an acid.
- Add a few drops of indicator. Note the colour.
- Do the same with a base.
- Add base drop by drop to the acid until you see a colour in-between that of steps 2 and 3.
- Use pH paper to get the pH.
- Keep adding drops of base, noting the pH each time. When the colour changes to that of the base, that is the end of the turning point. Remember the turning point involves a pH range.
39.
- Add phenophthalein to the acid. No colour results.
- Add base, drop by drop, until the first permanent pink colour results.
40.
Ca+2 ( alkaline earth metal) and Cl-1 ( halogen)produce CaCl2.
A calcium atom loses two electrons, one to each chlorine. The resulting bonds are ionic.
41.
V = I3R3
37.5 = 30I3
I3 = 1.25 A
Itop = 5 Ė 1.25 = 3.75 A
Vparallel = 37.5 = V1 + V2
V1= Itop*R1=3.75(4) =15 V
So V2 = 37.5-15 = 22.5 V
R2 = V2/Itop = 22.5/3.75 = 6 W.
OrÖ
1/Rp=1/[4+x) +1/30
But Rp = 37.5V/5A = 7.5W.
So 1/7.5=1/[4+x) +1/30
Multiply whole equation by 30(7.5)(4+x):
30(4+x) = 30(7.5) + 7.5(4+x)
120 + 30x = 225 + 30 + 7.5x
22.5x = 255 - 120
x = 6 W. Isnít math beautiful?!
42.
VIt = mcDt
6(I)(25)(60s/min)=200 g(4.19J/(gC))(38-24)
I = 1.30 A
43.
57g / [8(12)+18(1)]= 0.50 mole C8H18
2/0.5 = 16/x
x = 4 moles of CO2.
4 (44g/mole) = 176 g of CO2.

