1. C
2. similarities: both raise boiling points and lower melting points of water(physical prop)
differences in physical properties electrolytes conduct electricity; non electrolytes don’t
differences in chemical properties electrolytes react faster than non electrolytes; only electrolytes form ions; Nonelectrolytes are less corrosive
3. If lightning strikes water, it will conduct electricity and possibly electrocute you.
4. They lower the freezing point of water, so electrolytes can be added to slippery surfaces(roads, sidewalks). Less corrosive nonelectrolytes can be added to windshield fluid so it does not freeze.
5.
2 Br- à Br2 + 2e-
K+ + 1e- à K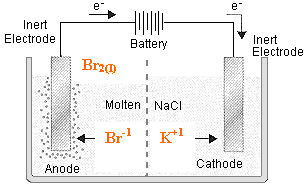
6. Wood alcohol (CH4O) is a non electrolyte. Write an equation to represent what happens when it dissolves in water.
CH4O(l) -->CH4O(aq)
7. Potassium chloride (KCl) is a salt. Write an equation to represent what happens when it dissolves in water.
KCl(s) -->K+(aq) + Cl-(aq)