More Pretest Questions
1.†††††††† Given:†† 1 molecule of nitrogen gas reacts with 3 molecules of hydrogen gas to produce 2 molecules of nitrogen trihydride gas.
 Write a chemical
equation for the above, and then draw what it represents using:
Write a chemical
equation for the above, and then draw what it represents using:
for each N atom, and
![]()
for each H atom.
2. One polyatomic that we have not studied is thiocyanate, SCN-1. Now that you know its name and charge, what is the formula of calcium thiocyanate?
3. What is the charge of Zn in ZnSO4?
4. What is the charge of the NO2 ion in Al(NO2)3?
5. Translate into a word equation:
Cl2(g)
+ 2 NaBr(aq) ![]() 2 NaCl(aq) + Br2(l)
2 NaCl(aq) + Br2(l)
6. Balance:
a. P4O10
+ H2O![]() H3PO4
H3PO4
b. Cu
+ HNO3 ![]() Cu(NO3)2 + NO + H2O
Cu(NO3)2 + NO + H2O
Solutions:
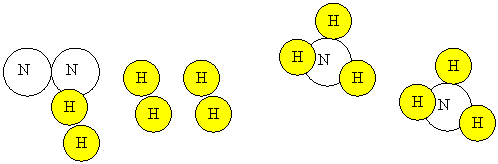
1.
N2(g)+ 3 H2(g)
![]() †2NH3(g)†††††††††††††††††††††††††††††††††††††††
†2NH3(g)††††††††††††††††††††††††††††††††††††††† ![]()
Note that
nitrogen and hydrogen gas consist of diatomic
molecules. Also note that each hydrogen can only be
attached to one atom in NH3.
2.
Ca+2 needs two SCN-1 in order to end up with a total
charge of 0. The correct formula is Ca(SCN)2.
3.
Since sulfate is Ė2,
then x + (-2) = 0, so x = 2. Zn is +2. Interestingly, although Zn is
technically a transition metal, its charge is always +2. So chemists donít
bother using the Roman numeral for zinc. Only two other transition metals are
that inflexible: silver, which is always +1; and cadmium, which is always +2.
4.
Since Alís charge is
known to be +3, then 3 + 3x = 0. x= -1, which is nitriteís charge. Nitrite has
the same charge as nitrate, NO3-1.
5.
One mole of chlorine
gas reacts with 2 moles of aqueous sodium bromide to produce 2 moles of aqueous
sodium chloride and 1 mole of liquid bromine.
P4O10
+ 6 H2O![]() †4 H3PO4
†4 H3PO4
6.
3 Cu +† 8 HNO3 ![]() 3 Cu(NO3)2 + 2 NO + 4 H2O
3 Cu(NO3)2 + 2 NO + 4 H2O
†††††††††††