p110
solutions
Exercises
1. The following table gives the colours of the indicator phenol red in solutions whose pH values vary from 0 to 14.
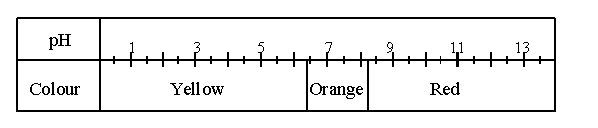
A few drops of this indicator are added to a basic solution.
What colour does the phenol red become?
2.
|
pH Scale |
2 |
3 |
4 |
5 |
6 |
7 |
8 |
9 |
10 |
11 |
12 |
||
|
Indicator 1 |
Yellow |
Green |
Blue |
||||||||||
|
Indicator 2 |
Colourless |
Pink |
Fuchsia |
||||||||||
|
Indicator 3 |
Red |
|
Yellow |
||||||||||
|
Indicator 4 |
Red |
|
Yellow |
Green |
|||||||||
You are given a solution and told it is neutral. You would like to check if this is true.
Which of the indicators in the table will you use? (pick only one)
Indicator
1; it has the narrowest turning point
3.
|
pH Scale |
2 |
3 |
4 |
5 |
6 |
7 |
8 |
9 |
10 |
11 |
12 |
||
|
Indicator 1 |
Yellow |
Green |
Blue |
||||||||||
|
Indicator 2 |
Colourless |
Pink |
Fuchsia |
||||||||||
|
Indicator 3 |
Red |
|
Yellow |
||||||||||
|
Indicator 4 |
Red |
|
Yellow |
Green |
|||||||||
The pH of a given solution is unknown. Indicators 1 and 3 turn yellow in this solution.
What colour will indicator 4 become in this solution?
orange
4. Below is the colour chart for an indicator.
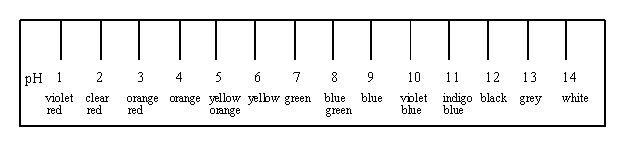
Maria carries out the following experiment : she numbers four test tubes 1 to 4 and into each adds 2 mL of the following substances and two drops of the indicator.
|
EXPERIMENT |
RESULTS |
|
|
Test-tube |
Substances |
Colours |
|
N° 1 |
2 mL of Drano solution |
indigo-blue |
|
N° 2 |
2 mL of vinegar |
red |
|
N° 3 |
2 mL of soft drink |
orange |
|
N° 4 |
2 mL of sodium bicarbonate solution |
blue green |
List the four test-tubes from least to most acidic.
1,4,3,2
5. The following table gives the colours of two indicators (A and B) when they are added to solutions with different pH values.
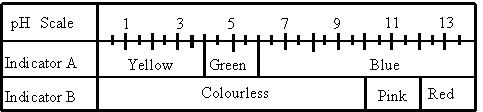
After adding a few drops of each indicator to a colourless solution of unknown pH, a student noticed that this solution turned blue.
What is the pH range of this solution?
6
to 10 ( remember: we have a mixture of the two indicators)
6. The following table gives the colour of a universal
indicator when it is added to solutions with different pH values.
|
pH |
1 |
3 |
5 |
7 |
9 |
11 |
13 |
Colour |
Red |
|
Yellow |
Green |
Turquoise |
Blue |
Purple |
In order to determine the nature of a solution,
a student added a few drops of this universal indicator to a sample of the
solution. The sample turned purple.
Was
the student a given a strong base? Explain.
Yes. The
higher the pH, the stronger the base.
7. a. Give the turning points for each of the
following:
1) Methyl orange: 3.2 to about 4.7
|
pH |
1 |
2 |
3 |
4 |
5 |
6 |
7 |
8 |
9 |
10 |
11 |
12 |
13 |
14 |
||
|
|
Red |
Orange |
Yellow |
|||||||||||||
2) Bromothymol blue: 6.3 to 7.8
|
pH |
1 |
2 |
3 |
4 |
5 |
6 |
7 |
8 |
9 |
10 |
11 |
12 |
13 |
14 |
||
|
|
Yellow |
Green |
Blue |
|||||||||||||
b. If you had
a strongly acidic solution with bromothymol blue indicator, and you slowly
added base, what colour changes would you see?
Yellow to green to blue.
c. In 7(b), suppose you had created a
strongly basic solution. How would you create a green solution?
Slowly add acid.