Chemisty 534
Pretest 3.3
Pretest: Redox Reactions (Questions and Solutions)
1. Determine whether the following statements are true or false.
____F__ a) Each hydrogen in NaH and HCl has an oxidation number of +1.
In metal hydrides, H =-1.
____T__ b) To calculate the oxidation numbers of the atoms in a polyatomic ion, the charge of
the ion must be used.
___T___ c) The oxygen in H2O has an oxidation number of –2.
___F__ d) The oxidation number for nitrogen in a molecule is always +5.
N can be : 0, 2,+3,-3, 4, 5 (don’t memorize this)
___T__e) A + eà A-1 is a reduction
2. Determine the oxidation number for each atom in the following molecules and find the total contribution by the atom.
a) AlCl3
O.N. |
Total contribution |
|
Al |
3 |
3 |
Cl |
-1 |
-3 |
b) OCl-1
O.N. |
Total contribution |
|
O |
-2 |
-2 |
Cl |
1 |
1 |
c) Mg+2
O.N. |
Total contribution |
|
Mg |
2 |
2 |
d) KClO3
O.N. |
Total contribution |
|
K |
1 |
1 |
Cl |
5 |
5 |
O |
-2 |
-6 |
3. Given the unbalanced equation:
FeCl3 + SnCl2 à SnCl4 + FeCl2
- Identify what is being oxidized and what is being reduced.
Fe+3-->Fe+2 : reduction
Sn+2--> Sn+4 : oxidation
- Write the two half-reactions
Fe+3 + 1e --> Fe+2
Sn+2 --> Sn+4 + 2e
4. Given the unbalanced equation:
HNO3 + H2S à NO + S + H2O
Identify what is being oxidized and what is being reduced.
N: reduced from 5 to 2
S: oxidized from -2 to 0
5. Given the unbalanced equation:
Br2 à BrO3 -1 + Br -1
Identify what is being oxidized and what is being reduced.
Br2 is getting oxidized from 0 to +5 in BrO3 -1. But Br2 is also getting reduced from 0 to –1 when it bcomes Br -1
6. a) Write two half reactions for the following reaction:
3 Zn + 2 Au+3 à 2 Au + 3 Zn+2.
a) Write two half reactions for the following:
3 Zn + 2 Au+3à 2 Au + 3 Zn+2.
Znà2e + Zn+2.
3e + Au+3 à 2 Au
b) Which would serve as the anode in an electrochemical cell?
The anode is where oxidation occurs(LEO) so that would be Zn.
c) Which substance would be the oxidizing agent?
The oxidizing agent is the one that causes oxidation of another substance and which steals electrons, and which is itself reduced, so it would be Au+3. Notice if you say Au, it will be wrong.Au and Au+3 are night and day, like good and evil, like rap and jazz :)
d) What ionic component would complete the circuit while maintaining the voltage?
the salt bridge.
e) Why are Zn, Ca and Mg all good reducing agents?
They are in relatively low oxidation states, lower than the usual +2 for them.
f) If the bridge had KCl which ion would move towards the gold electrode and which electrode would gain mass?
The cation(K+) would move towards gold; the cation is attracted to the cathode(site of reduction) by the (-) ions left behind by the reacting Au+3.
7. Balance the following redox reaction occurring in a basic solution by means of the half reaction method.
S-2 + NO3-1 à NO2 + SO2
4OH-1 + S-2 à SO2 + 6e + 2 H2O (notice that the sulfur is going from -2 up to +4)
NO3-1 + 1e + H2O àNO2 + 2 OH-1 (notice that the nitrogen is going down from +5 to +4)
Sum:
S-2 + 6 NO3-1 + 4 H2O à SO2 + 6 NO2 + 8 OH-1
8. Balance the following redox reaction occurring in an acidic solution by means of the half reaction method.
HNO3 + H2S à NO + S + H2O
3H+1 + 3e + HNO3 à NO + 2H2O
H2S àS + 2e + 2H+1
Balance the equation
(multiply 1st half-reaction by 2 and 2nd by 3)
2 HNO3+ 3 H2S -->2 NO + 4 H2O +3 S
9.
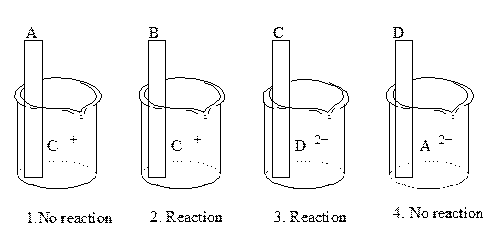
Which of the above metals is the strongest reducing agent? Rank them all from strongest to weakest. Do likewise for reducing agents.
In those that react:
(2) B + C+ --> B+ + C
so B > C as a reducing agent
(3) C + D2+ --> C + + D
so C > D as a reducing agent
In those that don't react, imagine that we're failing to get a a reaction becuase we are trying to get the products of the following to react
(1)A2++ C--> C + + A
so C > A as a reducing agent
(4)A + D2+--> A2+ + D
so A> D as a reducing agent
Overall : B > C> A> D as reducing agents:
D2+ > A2+ > C + > B+
as oxidizing agents
Flashback Themes: Avogadro; Pressure
9. Two tanks filled with gas are under the same conditions of temperature and pressure.
One is filled with hydrogen H2 and the other with nitrogen N2.
According to Avogadro's law, which of the following statements is true?
A) |
Nitrogen molecules are more numerous than hydrogen molecules. |
B) |
Nitrogen molecules are as numerous as hydrogen molecules. |
C) |
The two tanks contain equal masses of gases. |
D) |
Nitrogen molecules are less numerous than hydrogen molecules. |
10. Volume, pressure, and temperature are the three variables that determine the state of a certain mass of gas.
Boyle's Law describes the relationship between the pressure and the volume of a fixed mass of gas at constant temperature.
What is the relationship?
A) |
The pressure is directly proportional to the volume. |
B) |
The pressure is directly proportional to the square of the volume. |
C) |
The ratio of the pressure over the volume is constant. |
D) |
The product of the pressure and the volume is constant. |
11. Two identical balloons were filled at the same temperature. The first contains 10 g of nitrogen gas, N2, and the second, 12 g of carbon dioxide, CO2.
In which balloon is the pressure greater?
Explain your answer.
Nitrogen gas exerts the most pressure because it has the largest number of moles of molecules (10g/[28g/mole] ) = 0.36 mol > 0.27 mol =12/44).
12. What family of the periodic family has exactly three electrons occupying the p orbitals?
nitrogen family
13. What is the electron configuration for Mn?
1s2 2s2 2p6 3s2 3p6 4s2 3d5
14. Every time Sporcaccione bends down, he lets out digestive gas. What gas law is related to this phenomenon? Explain
Boyle’s Law. Bending down squeezes and temporarily reduces the volume of his large intestine, increasing pressure. But because there is an outlet at one end of the intestine, digestive gas, with its increasing collisions, finds its way out of Sporcaccione’s body.