Exercises for Rate of Combustion ( Solutions)
1. Write a balanced equation for the combustion of pentane. Pentane is C5 H12. Identify the fuel and the oxide, and include heat on the appropriate side of the equation.
C5 H12+ 8 O2 ŗ5 CO2 + 6 H2O
+ heat
††††††††††† Fuel: C5 H12††††††††††††††††† oxides: CO2 + H2O
2. Draw a reaction profile in which H reactants = 100 kJ, the activation energy is 50 kJ, and DH = -25 kJ.

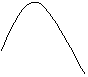 †††††††† 150
†††††††† 150
††††††††††††††††††††††† E (kJ)
††††††††† 100![]()
![]() ††††††††††††††††††††††††††††††††††† 75
††††††††††††††††††††††††††††††††††† 75
††††††††††† Note:††† Ae = Hmax Ė Hreactants
=150 - 100 = 50 kJ.
††††††††††††††††††††††† DH = Hproducts Ė Hreactants = 75-100 = -25 kJ
†††††††††††††††††††††††
3. Which of the following is not absolutely necessary for combustion to take place?
A.††††††† a fuel††††††††††††††††††††††††††††††††††††††† B.†††††††† oxygen
C.††††††† match†††††††††††††††††††††††††††††††††††††† D. †††††† a sufficiently high temperature
Why? A lighter, spark or surrounding air(as
in spontaneous combustion) can provide a sufficiently high temp. It does not
have to be a match.
4. Carbon burns in air to produce CO2 and H2O. Under what conditions would you expect the fastest rate of combustion?
A.††††††† Chunks of carbon are heated and allowed to burn naturally in air.††††††††††
B. Forcing hot air over the carbon as it burns.
C. Powdering the carbon and allowing it to burn naturally.
D.
Powdering the carbon
and forcing hot air over it.
(the answer is D because youíre
increasing surface area through powdering, increasing oxygen by forcing air,
and since itís hot air, youíre raising the temperature.)
5. How does the foam from a fire extinguisher help put out the fire?
It
prevents oxygen from reaching the fuel, thus smothering the fire. The foam
itself is not flammable.
6. A cook started a fire by forgetting about the oil he was warming. He then pulled the pan off the stove and threw baking soda in the pan.
Explain why the cook acted the way he did. Why did he not use water?
Oil floats on water, and does not mix with it. This will
prevent the water from cooling the oil effectively, and above the water the oil
will continue to encounter oxygen. Since it stays hot and keeps getting oxygen,
the oil keeps burning.
Baking soda, on the other hand, acts like the fire
extinguisher in question 5.
1. Olive oil has a lower kindling point than corn oil. Which one is the more practical cooking oil?
Corn oil is more practical because it does not burn as
easily (higher kindling point). By reaching a higher temperature before
burning, corn oil helps food cook faster.
8.††††††††††† According to the manufacturer, the average rate of combustion of a certain
††††††††††† type of candle made of paraffin, C25H52 , is 8.33 x10-4 mol/min. This type of
††††††††††† candle is sold in four sizes: 25, 50, 75 or 100 g. You wish to use only one
††††††††††† candle of this type to provide 4 continuous hours of light. What is the smallest
††††††††††† one you can buy for this purpose ?
††††††††††† In 4 hours = 240 minutes,
[ 8.33 x10-4 mol/min][240 minutes] = 0.1999200000 moles
will burn.
††††††††††† 0.1999200000
moles(25*12+52*1 g/mole) =† 70.4 g (with sig
figs = 7 X 101 g) will burn.
††††††††††† So you need to buy at least a 75 g candle to last you four hours. Thatís one long bath by candle light!
9.†††††††† From a molecular point of view, why does a combustion reaction need to reach a kindling point before a fire starts?
Only at that temperature will fuel molecules and oxygen
molecules be able to collide with enough energy to
create oxides.
10.†††††† From a molecular point of view, why does surface area increase the rate of a reaction?
††††††††††† Molecules
are only exposed at the surface. Those below the surface do not immediately
collide with the other reactant. Having more surface exposes more molecules to
a reactant at a given instant, increasing the rate. Thatís why if youíre in a
rush to make mashed potatoes, you should cut open the potatoes before tossing
them into the water. Of course, practically speaking, one cut will suffice
since too many cuts will create a mess in boiling water.
11.†††††† Ae =133 Ė83 kJ =50. kJ