1.†††††††† Significant Figures
What are significant figures?
They are digits that are obtained or meaningfully derived from a
measurement. They include the single estimated figure that is part of every
measurement. For example suppose that you weigh some sodium chloride.
The electronic balance reads 2.0 g. Itís important that you record the mass as
2.0 g. The balance is telling you (at the very best; the uncertainty can be
even greater.) that the actual mass is anywhere between 1.95 and 2.04 grams.
But if you record 2g, you are telling your reader that the mass lies anywhere
between 1.5 and 2.4 grams: an injustice to the balance!
When performing calculations, significant figures must also be
respected. For instance if the 2.0 g of NaCl is dissolved in 60.0 mL of water,
the calculator yields a concentration of 2.0/0.060 L = 33.3333333g /L. Where do
you round off? The correct answer is 33g/L.
When dividing or multiplying, the answer must have as many significant
figures as contained in the original measurement with the least number of
significant figures.
Not convinced? Remember when we measure 2.0, we donít know what the
third decimal really is. Similarly, for 60.0 mL the actual volume may be
anywhere from 59.95 to 60.04. So letís look at some possibilities.
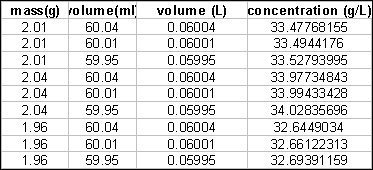
The results range from 32.6 to 34.0. Clearly the decimal place is
meaningless. 33 g/L is the answer that reflects the accuracy of our
measurements.
Rules for Determining Significant Figures.
1.
All
non-zero digits are significant. In any measurement, the last significant
figure is an estimate but is still significant.
|
Example |
Number of Significant Figures |
|
2365 |
4 |
|
1.3365 |
5 |
|
11 |
2 |
2.
Leading
zeros (0ís before the nonzero digits) are NOT significant.
|
Examples |
Number of Significant Figures |
|
0.005333 |
4 |
|
0.3 |
1 |
|
0.000010 |
2 |
If you find this illogical, remember that the
above numbers can be converted into scientific notation. For example 0.3 = 3 X10-1 has one significant
figure.
3.
Captive
zeros (those between nonzero digits) are significant.
|
Examples |
Number of Significant Figures |
|
0.005003 |
4 |
|
0.301 |
3 |
|
3005 |
4 |
4.
Trailing
zeros (those after nonzero digits) are significant only if the measurement
contains a decimal.
|
Examples |
Number of Significant
Figures |
|
50000 |
1 |
|
50000. |
5 |
|
7.000 |
4 |
5.
Exact
numbers and irrational numbers have an infinite number of significant numbers.
|
Examples |
Number of Significant
Figures |
|
p |
infinite |
|
The 2 in C = 2 pr |
infinite |
|
3 people |
infinite |
6.†††††††† After
carrying out a multiplication or division, the answer must have as many
significant figures as contained in the original measurement with the least
number of significant figures.
|
Examples |
Answer |
Number of Sig Figs in Answer |
|
100./25 |
4.0 |
2 |
|
622.3 *3.5 |
2.2 X 103 |
2 |
|
3.65 per 4 people |
0.91 per person |
2 (
note the 2 people have an infinite number of sig figs and do not act as the
limiting measurement |
7.†††††††† For
adding and subtracting, the answer must have the same number of decimal places
as the least precise measurement
( the one with the least decimal
places.)
|
Examples |
Answer |
|
23.91 + 11.999 |
35.91 |
|
11110.2 + .1333 |
11110.3 |
|
|
|
Important:††††† The rules for significant figures should be applied only to
the final answer. That implies that all decimal places should be retained
in the middle of a calculation.
8.†††††††† When taking the logarithm of a number, the answer gains a significant figure. Conversely when a measurement is applied as an exponent, the answer loses a significant figure. The reason is that in the latter case there is a greater propagation of error due to the exponent.
|
Examples |
Answer |
|
pH = -log(0.045) |
pH =1.35 |
|
10-6.34 = |
4.6 X 10-7 Note that for a pH measurement of 6.34 +/-
0.01, an value of 6.33 leads to a concentration of 4.68 X 10-7
whereas 4.57 X 10-7 The third figure is clearly meaningless |
|
|
|
Siginificant
Figures Exercise
1.†††††††† What is the correct number of significant figures in the following measurements?
a. 2.0004 cm
b. 300. cm††††††††††† (there is a deliberate point after the last zero)
c. 300 m
d. There are about 6 billion people on earth.
e. 1.9900 ml
f.††††††††† ††††† ††††† 2.00 X 104 kg
2.†††††††† The mass of an empty can is 61 ![]() grams. Then 30
grams. Then 30 ![]() grams of water are added to the can. What is the lowest
possible total mass for the can and water? The highest?
grams of water are added to the can. What is the lowest
possible total mass for the can and water? The highest?
3.†††††††† A student observed that the temperature of 100.0 ml of water with a known density of 1.0 g/ml increased from 10.5 oC to 22.8 oC. Express the amount of heat absorbed by the water in kJ with the correct number of significant figures. Use c = 4.19 J/(goC).
4.†††††††† A gas sample contains 0.233 moles of He and 0.35 moles of H2. What is the total number of moles of gas in the sample, expressed with the correct number of sig- figs?
5.†††††††† H2 with a molar mass of† 2(1.0797) g/mole consumes 8.0 grams of sodium, according to the following reaction:
2 Na (s)†††††††††††† +††††††††† H2(g)†††††† ŗ††††††††††††††††††† 2 NaH(s)
How many grams of sodium hydride, NaH will be produced? Express with the correct number of significant figures.
6.†††††††† The initial concentration of ozone, O3 is 5.0 moles/L.† If the equilibrium concentration of oxygen is 3.0 moles/L, what is the value of K, expressed with the correct number of sig figs?
7.†††††††† In a lab, our measurements for the height of a tube ranged from 5.5 cm to 6.8 cm. The least accurate concentrations of the solutions used was 0.0010 M. Assuming that the value for K( an equilibrium constant) should have been expressed with just as many significant figures as in the above numbers, how should Peter have expressed the average of the following 4 values for K?
|
96.37754 |
|
87.05914 |
|
126.1661 |
|
230.3015 |