Solutions to January Exam review #4
1. B ( Remember the graphical relationship is hyperbolic)
2. D (should read 70 g/mole) Equal volumes of
ideal gases exposed to the same conditions of pressure and temperature contain
the same number of molecules and so have the same number of moles. 1.4/28 =
0.05 moles. 3.5/0.05=70.
3. A
4. D
5. B
6. C
7. C (donít forget to convert to K)
8. A...
9.
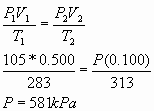
10.a The ratio is 2:1, so there are 3.5 X 2 = 7 moles
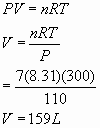
b.
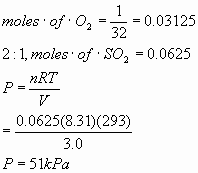
Copyright ©2009
Created:April/6/1996;
Updated: Dec/2/2009