Solubility : Why Crown Ethers are Special
 To
understand solubility we should first distinguish between polar and and non-polar solvents. Polar solvents have a strong
dipole, which is created by the type of bonds a molecule has and by its geometry.
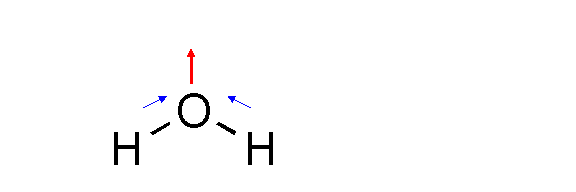
In water, for example, oxygen is more electronegative than hydrogen, meaning
that the O pulls electrons closer to itself from each hydrogen atom (shown in
blue) But there is an angle of 104.5° between
the two OH bonds. The resultant of these two forces (shown in red) is what
gives water a strong dipole—in other words, there is
an overall positive and negative charge to the water molecule, rendering it a polar solvent.
To
understand solubility we should first distinguish between polar and and non-polar solvents. Polar solvents have a strong
dipole, which is created by the type of bonds a molecule has and by its geometry.
In water, for example, oxygen is more electronegative than hydrogen, meaning
that the O pulls electrons closer to itself from each hydrogen atom (shown in
blue) But there is an angle of 104.5° between
the two OH bonds. The resultant of these two forces (shown in red) is what
gives water a strong dipole—in other words, there is
an overall positive and negative charge to the water molecule, rendering it a polar solvent.
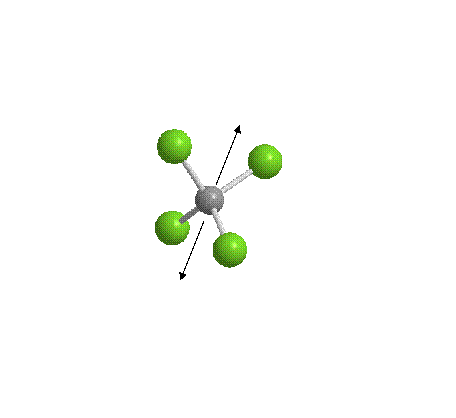
CCl4 has 4 polar C-Cl bonds but no overall dipole because the four forces cancel out. I’ve shown two black arrows, each representing the resultant of two forces. CCl4 is known as a non-polar solvent.
If CCl4 and water are mixed, there is an attraction between the different molecules but although it is nothing to be scoffed at, it is far weaker than the forces between water molecules. Water molecules form hydrogen bonds between themselves as the oxygen from one molecule will be attracted to a hydrogen atom from another molecule. So water molecules will hang around with their own kind and will not mix with CCl4. Generally this is true, leading to the rule that like dissolves like.
I2 is also non-polar and it will dissolve in CCl4. Neither of these will dissolve ionic substances because they cannot prevent the ions from reassociating. Water, on the other hand can effectively shield many (but not all)ions through its strong dipole which attracts both positive and negative ions.
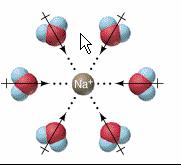
The stability of these arrangements between ions and water molecules are what compensate for the energy that must go into breaking the bonds between the ions of a solid crystal.
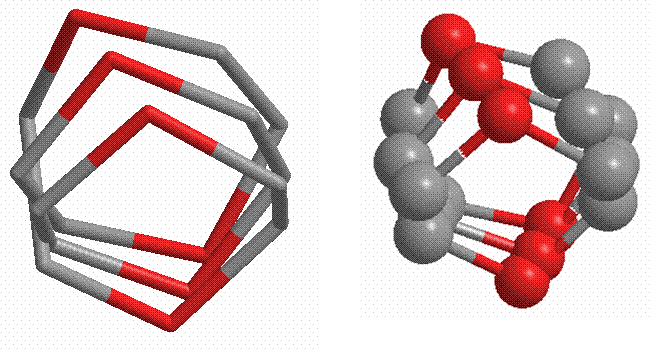
Can a non polar substance ever have hope of dissolving ions? In 1969 French chemist Jean Marie Lehn showed that the central cavity of a crown ether (a non-polar organic discovered by Charles Pederson in 1967) could attract a metal ion. The diagrams below reveal the structure of 18-Crown-6 ether (C12H24O6). For clarity’s sake, the hydrogen atoms are not shown in either diagram, and the structure to the left ignores the balls to better reveal the overall closed spiral nature of the molecule
The space within this specific molecule is ideal for accommodating K+1. This means that even when a non-polar solvent like benzene is mixed with 18-Crown-6 ether, it will still dissolve KMnO4. The free permanganate ion can be used to oxidize alkenes. To dissolve smaller ions such as Na+ and Li+, correspondingly simpler crown ethers can be used such as 15-Crown-5 and 12-Crown-4, respectively.
These types of compounds have been modified into 3-D molecules known as cryptates which accommodate specific molecules, similar to the way enzymes operate. Such molecules have been used not only as catalysts but as carriers of substances across biological membranes.
References: