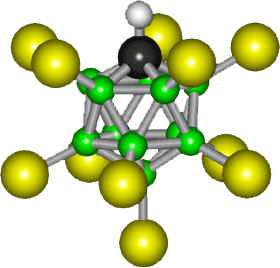
Discovered in 2004, the world's strongest acid is carborane acid with the formula
H(CHB11Cl11). Its anionic part (the part without the acidic proton)looks
like this:

|
http://en.wikipedia.org/wiki/Carborane Enrico: See footnote 2, which tries to explain (ineffectively) that the proton is not shown. It depends on the phase. In the solid state, the acid is a polymer with Cl---H+---Cl bridges. In the gas phase, the acid is a monomer with the proton bridging the 12 and 7 positions (farthest from CH) although the 7,8 isomer is probably also present. In solution, it will typically be ionized to give a disolvated proton, H(solvent)2(+), and the free a CHB11Cl11- anion shown in the picture. See JACS(Journal of Amercian Chemical Society) 2006, p3160. Perhaps you'd like to update Wiki? Sincerely, Chris Reed |
Page Maintained by Enrico Uva
Technical Asistance: C. Frizzell; and M.Pololos, D.Verrillo and K.Papoulias at EMSB
Comments: euva@retired.ca
or
Comments: euva@prof.emsb.qc.ca
Copyright ©2006
A violation of sig figs!!