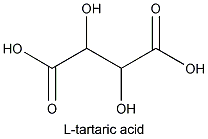
 m of tartaric acid is L- or
(+)tartaric acid.
m of tartaric acid is L- or
(+)tartaric acid.Tartaric Acid
The most abundant naturally occurring for m of tartaric acid is L- or
(+)tartaric acid.
m of tartaric acid is L- or
(+)tartaric acid.
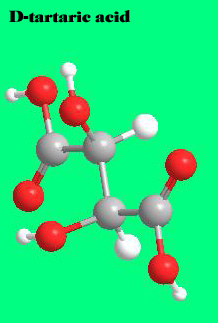
It has the following 3d structure:

This acid is not only found in grapes but in a wide variety of fruits. It is fairly acidic: a 0.1 N solution will have a pH of 2.2.
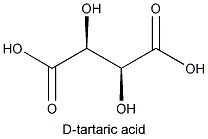
The molecule that constitutes its mirror image is less common in nature: D- or (-) tartaric acid. The microorganism Penicillium notatum ,among others, will convert a mixture of the two images into the latter form.
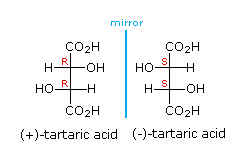
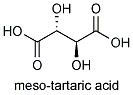
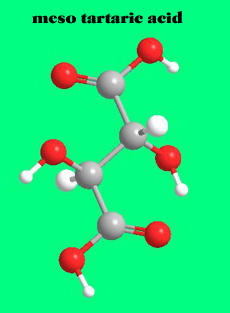
If the OH and H group on one of the chiral carbons are inverted, then the molecule will have an internal plane of symmetry. Such a molecule , meso tartaric acid, no longer exists in two forms; both images would be superimposable.
KC4H5O6 + NaHCO3 --> CO2 + H2O + KC4H4O6Na
My wife once tried to substitute baking powder with baking soda in a recipe that contained very fewacidic ingredients. The result: the most bitter cookies I've ever tasted. The bitterness was, as you guessed, due to unreacted NaHCO3, which is a base.
Data from:
Merck Index 12th Edition.