The Models of the Atom
All life, whether in the form of trees, whales, mushrooms, bacteria or amoebas , consists of cells. Similarly, all matter, whether in the form of aspirin, gold, vitamins, air or minerals, consists of atoms, which, regardless of size, are made up of the same basic units. This took us thousands of years to realize, and the present chapter is a journey through that history, one that eventually gave us enough understanding and power to mimic, harm and repair nature in ways never before possible.
The next five paragraphs are taken, almost word for word, from the Britannica DVD, 2000 edition.
Aristotle emphasized that nature consisted of four elements: air, earth, fire, and water. He thought these are bearers of fundamental properties, dryness and heat being associated with fire, heat and moisture with air, moisture and cold with water, and cold and dryness with earth. He did not believe in discontinuous or separate atoms but felt that matter was continuous.
Democritus (b. c. 460 BC; d. c. 370 BC) postulated the existence of invisible atoms, characterized only by quantitative properties: size, shape, and motion. Imagine these atoms as indivisible spheres, the smallest pieces of an element that still behave like the entire chunk of matter.
Dalton (b. Sept. 6, 1766, England; d. July 27, 1844) deduced the law of multiple proportions, which stated that when two elements form more than one compound by combining in more than one proportion by weight, the weight of one element in one of the compounds is in simple, integer ratios to its weights in the other compounds. For example, Dalton knew that oxygen and carbon could combine to form two different compounds and that carbon dioxide (CO2) contains twice as much oxygen by weight as carbon monoxide (CO). In this case, the ratio of oxygen in one compound to the amount of oxygen in the other is the simple integer ratio 2:1. Although Dalton called his theory "modern" to differentiate it from Democritus' philosophy, he retained the Greek term atom to honour the ancients. Also, Dalton gave us more insight into molecules, but his idea of the atom was not that different from that of Democritus: he still imagined atoms as tiny “bowling balls.”
J.J. Thomson (b. Dec. 18,
1856, England d. Aug. 30, 1940,) held that atoms are uniform
spheres of positively charged matter in which electrons are embedded. He
realized that electrons existed by improving Crooke’s tube, a tube that
contained a small amount of gas through which electricity was passed. Crooke
had noticed that the rays inside the tube were bent by a magnet, something that
ordinary light does not do. By
developing a better vacuum, Thomson showed that these same rays also bend
towards the positive plate of a battery, no matter what gas produces them.
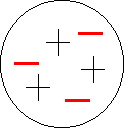
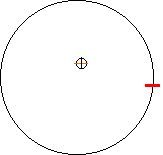
Thomson concluded that these “cathode rays” were basic particles found in all elements. He went on to propose what is popularly known as the plum-pudding model of the atom. In other words he thought that atoms were not indivisible spheres, but positive spheres with negative electrons embedded in them. It had to be abandoned (1911) on both theoretical and experimental grounds in favour of the Rutherford atomic model.

Ernest Rutherford. (b. Aug. 30, 1871, New Zealand; d. Oct. 19, 1937, England)
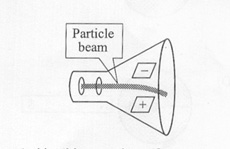
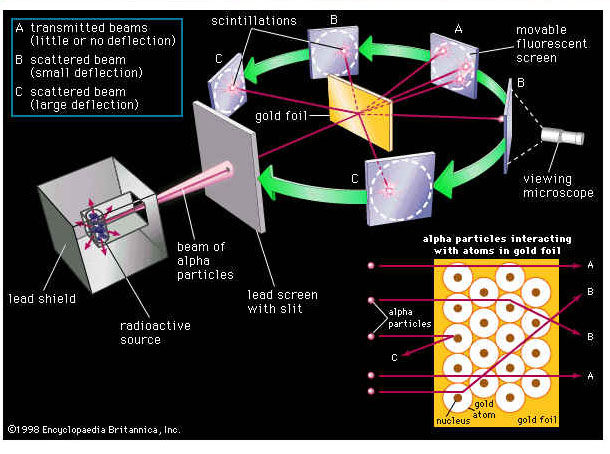
The model described the atom as a tiny, dense, positively charged core called a nucleus, in which nearly all the mass is concentrated, around which the light, negative constituents, called electrons, circulate at some distance, much like planets revolving around the Sun. The Rutherford atomic model has been alternatively called the nuclear atom, or the planetary model of the atom. The young physicists beamed alpha particles through gold foil and detected them as flashes of light or scintillations on a screen. The gold foil was only 0.00004 centimeter thick. Most of the alpha particles went straight through the foil, but some were deflected by the foil and hit a spot on a screen placed off to one side. Geiger and Marsden found that about
one in 20,000 alpha particles had been deflected 45o or more. Rutherford asked why so many alpha particles passed through the gold foil while a few were deflected so greatly. "It was almost as incredible as if you fired a 15-inch shell at a piece of tissue paper, and it came back to hit you," Rutherford said later. "On consideration, I realized that this scattering backwards must be the result of a single collision, and when I made calculations I saw that it was impossible to get anything of that order of magnitude unless you took a system in which the greater part of the mass of the atom was concentrated in a minute nucleus. It was then that I had the idea of an atom with a minute massive center carrying a charge."
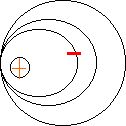
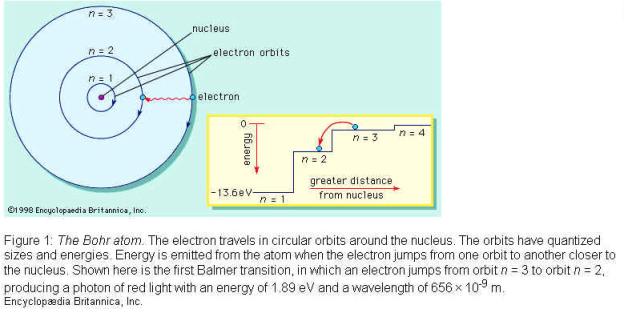
Niels Bohr In 1913 he proposed his quantized shell model of the atom to explain how electrons can have stable orbits around the nucleus. The motion of the electrons in the Rutherford model was unstable because, according to classical mechanics and electromagnetic theory, any charged particle moving on a curved path emits electromagnetic radiation; thus, the electrons would lose energy and spiral into the nucleus. To remedy the stability problem, Bohr modified the Rutherford model by requiring that the electrons move in orbits of fixed size and energy. The energy of an electron depends on the size of the orbit and is lower for smaller orbits. Radiation can occur only when the electron jumps from one orbit to another. The atom will be completely stable in the state with the smallest orbit, since there is no orbit of lower energy into which the electron can jump.

![]()
![]()

The Hydrogen Spectrum
See other spectra
(From
http://www.achilles.net/~jtalbot/data/elements/)
Summary
|
Model of the Atom |
Sketch |
Experimental Evidence |
Description of Model |
|
|
|
none |
Atoms are made up of discontinuous, indivisible spheres |
|
|
|
Some, but you’re not responsible for knowing it |
· An atom is divisible · Atoms of the same element are identical. · Atoms of different elements have different masses · Atoms can be rearranged to produce new substances. |
|
Thomson |
|
When electricity was passed through a small amount of gas, cathode rays(electrons) were produced. These were attracted to a positive plate. He also worked out the charge to mass ratio of these "rays" confirming that they were indeed particles. |
A neutral atom is made up of an equal number of positive and negative particles.(the plum-pudding model) |
|
Rutherford |
|
Alpha particles(+) were fired at a sheet of thin gold foil. Surprisingly a few particles bounced back—this was like toilet paper stopping rifle bullets. |
The nucleus of the atom is extremely small and positively charged. The rest of the atom is mostly empty space and it contains electrons. |
|
Bohr |
|
(436 only) Hydrogen gas spectrum consists of thin lines of red, green, blue and violet. |
Electrons can only occupy certain energy levels around the positive nucleus. |
|
Particle Glossary |
|||
|
Particle/Ray |
Other name |
Charge |
Origin |
|
electron |
Beta Particles, b, cathode rays |
Negative. [they’re attracted to (+)] |
From radioactive decay |
|
alpha particle |
helium nucleus, a |
positive (+2), [they’re attracted to (-)] |
From radioactive decay |
|
gamma rays, g |
|
none; they’re a form of energy and are not attracted to either positives or negatives. |
From radioactive decay |
|
proton |
|
+1 |
Basic particle of nucleus; defines the atom = number of protons =atomic number |
|
neutron |
|
neutral |
Basic particle of nucleus; changes the mass of an atom and creates different isotopes, but does not affect chemical properties |