Chemistry
534
Pretest
1.2 Show all work. Use
loose leaf. Solutions in blue
1. Consider
the first step in the industrial production of nitric acid:
4
NH3(g) + 5 O2(g) -----> 4 NO(g) + 6
H2O(g)
a. What is the most oxygen ( in liters at
STP) that could react with 68.0 g of NH3(g)?
68.0g/(17.0g/mole)
= 4.00 moles of NH3(g).
The ratio of NH3(g) to O2(g) is 4 to
5, so to completely react with 4.00 moles of NH3(g), 5.00 moles of
oxygen will be needed
At STP, 5.00 moles
of oxygen occupy 5.00 *22.4 = 112 L
b. In burning ammonia, the STP equivalent
of 224 L of oxygen were consumed. How many grams of NO must be contended with
at STP?
224 L/22.4 L =
10.0 moles of oxygen at STP.
The ratio of
oxygen to NO is 5 to 4, so 8.00 moles of NO will be created.
8.00 moles
(14.0+16.0 g/mole) = 240. g or 2.40 X 102
g of NO
2. Acetylene reacts with oxygen according to the
following equation.
2 C2H2(g) + 5 O2(g) ® 4 CO2(g) + 2 H2O(g)
|
In an oxyacetylene
blow torch, steel cylinders containing acetylene and oxygen are connected
through hoses that join together, and then connect to a nozzle. The mixture is combusted and produces a
flame capable of cutting through metal. |
|
When a steel cylinder of oxygen with a volume of 14.5
L was used to supply oxygen to an oxyacetylene torch, the pressure in the
oxygen cylinder changed from 2.080 ´ 103 kPa to 2.010 ´ 103 kPa. The temperature of both cylinders was 22.0°C
at the times of both pressure readings.
What mass of acetylene was
combusted from the other cylinder?
First find n1 for oxygen using PV = nRT:
n1
= P1V/RT = moles oxygen originally present.
n2
= P2V/RT = final moles oxygen present.
Amount
of oxygen consumed = n1 - n2 = P1V/RT - P2V/RT
= V/RT (P1 - P2) =14.5/(8.31*[22+273])(2080-2010)
=
0.414 moles O2
The ratio
of C2H2 to O2 is 2 to 5, so the amount of
acetylene that reacted with 0.414 moles
O2 is (2/5)* 0.414 = 0.1656 moles of C2H2.
The mass of
0.1656 moles of C2H2.= 0.1656 *(2 * 12.011+2 *1.00797)
g/mole = 4.31 g.
3. Use diagrams of molecules in cylinders
( with pistons) to show that if pressure is halved, volume doubles.

In the original
cylinder, the molecules are crowded: four collisions result. With half the
pressure, the molecules have just as much kinetic energy, but they are covering
more room in the same instant because there are less collisions between the
molecules and between the walls of the container.
4. Use PV = nRT to come up with four linear relationships between two variables. Mention the constant in each case.
· P vs T at constant n and constant volume
· P vs n at constant T and V
· V vs n at constant P and T
· V vs T at constant n and constant pressure
Only P vs V and T vs n are not linear.
5. What is the density of argon at –50.0o
C and 200. kPa?
PV = nRT
n/V = P/RT =
200/[(8.31*(-50.0 + 273)) = 0.108 moles/L = 0.108 moles*39.948(g/mole)/L = 4.31
g/L.
6. A student wants to triple the pressure
of an ideal gas, while decreasing the volume by a factor of 0.8 and increasing
the temperature from 200K to 250 K. If
there were 2 moles of gas originally in the gas tank, should he remove gas? Add
gas? Explain.
![]() P2 = 3P1; V2= 0.8V1;
P2 = 3P1; V2= 0.8V1;
![]()
n2 = 3.84 moles. So he should
add 3.84-2 = 1.84 more moles of gas to meet those conditions. With sig figs, the answer is rounded to 2
more moles needed. Note that there is only one sig fig in one of the
measurements.(2 moles)
7. If we had done the hydrogen lab on a
rainy day when atmospheric pressure is lower, how would it have affected the volume of hydrogen
collected?
Assuming
constant pressure, (Boyle's Law), with lower atmospheric pressure we would have
collected a greater volume of hydrogen. Would we have gotten more moles of
hydrogen? Of course not.
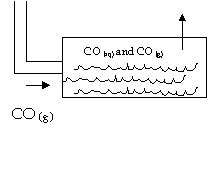
8. Carbon
monoxide (CO)can form from the following reaction: FeO + C --> Fe + CO
As
the gas forms, it is cooled to 20.0 C, and it first passes through water at the
same temperature, and then escapes into a lab that holds 125 000 L of air at
101.3 kPa. Will there be enough CO to kill someone if 378.3 g of FeO react?
Lethal
dose of CO is 0.1 % of total by volume.
At
20.0 C, 3.3 ml of CO dissolve in the water found in the tank.
378.3g/ ([55.8+15.9994]
g/mole) = 5.2688.. moles of FeO
Ratio of FeO to CO
is 1:1 so
Using PV = nRT we can find the volume of CO produced
PV
= nRT
(101.3kPa)V = (5.26888)(8.31kPaL/[molesK])(293K)
V = 126.64111 L
However,
3.3 ml of CO dissolves in the water tank (stays there)and so
V of CO in
the air = 126.64111L - 0.0033L
=126.638L
% CO in the air =
Volume of air in the lab
=
126.638L / 125 000 X 100%
=
0.101% > 0.100% = Lethal dose. With
sig figs answer = 0.10%
This reaction would release enough CO to be lethal.
9. How many significant figures are
contained in the measurement 0.002030 cm?
4 ( leading zeros are never significant; captiveones always are.
Trailing zero is OK here because of the decimal)
10. Click here for problem and solution.
