Rates of Reactions (continued)
1. Combustion and Activation Energy
· Combustion, as you recall, involves a reaction between a fuel and oxygen.
· The products of a combustion reaction always include an oxide that binds to a victimized atom within the fuel (we’re dramatizing here: the victim is simply an atom that lost electrons to the electron-hungry oxygen).
· If there is hydrogen within the molecules of the fuel, water will also be produced.
Example 1: Predict what will form from the combustion of the following:
|
Fuel |
Products of combustion |
Equation |
|
Aluminum ( Al) |
Al2O3 |
2 Al + 1.5 O2--> Al2O3 |
|
Methane (CH4) |
CO2 + H2O |
CH4 + 2O2-->
CO2 + 2H2O |
|
Gasoline ( C8H18) |
CO2 + H2O |
C8H18+
12.5O2--> 8CO2 + 9H2O |
|
coal (mixture of S + C) |
SO2 + CO2 |
S+ O2--> SO2
; C+ O2--> CO2 |
|
wood (mixture of [C6H10O5]n and [C10H12O3]n ) |
CO2 + H2O |
----------------------------------- |
· Because combustion forms very stable products, combustion reactions are exothermic.
Example 2: Draw a reaction profile (enthalpy versus reaction-progress) for a combustion reaction.
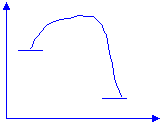
Enthalpy(kJ)
Progress of reaction
· Although combustion reactions eventually release heat, they also need heat to get started. The temperature at which oxygen molecules smash into fuel molecules with enough energy so that electrons are stolen and products are formed is known as the kindling point.
|
Substance |
Kindling Point
(oC) |
|
Paper |
232 |
|
Wood (varies with type) |
190-266 |
|
Cotton |
266 |
|
Methyl alcohol |
464 |
|
Natural gas(depends on composition) |
482-632 |
· The little bump in the graph on the previous page represents the amount of energy needed to reach the kindling point. Many non-combustion reactions also need a jump-start , and in general it is known as the activation energy, Ae. The activation energy can be calculated by :
Ae = H maximum – H reactants
Example 3. Calculate the activation energy for the following:

 350 kJ
350 kJ
![]() 300 kJ
300 kJ
![]() 200 kJ
200 kJ
Activation energy = Ae = 350-300 kJ = 50 kJ
2.
Rates of
Combustion
The first three factors that control the rate of combustion are components of the fire triangle. They are heat, oxygen and fuel.
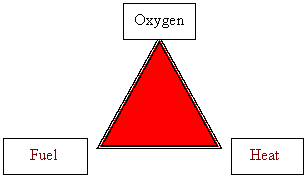 If
any of the three components is missing, the rate will be zero; in other words,
the fire will stop. For obvious reasons, firemen are taught the fire triangle:
If
any of the three components is missing, the rate will be zero; in other words,
the fire will stop. For obvious reasons, firemen are taught the fire triangle:
Water puts out most fires because it absorbs a great deal of heat with its high specific heat capacity, so it cools the fire.
CO2 extinguishers which release carbon dioxide by reacting Na2CO3 with H2SO4,( or by having liquid carbon dioxide under pressure)
smother the fire and deprive it of oxygen.
In fighting fires it is essential to prevent a fire from reaching areas rich in fuels such as oil furnaces. Any gas lines leading to the burning area must be automatically shut off as the alarm is triggered. For more firefighting details see this link.
The following affect the rate of combustion, or simply put, how fast something burns. Note that 3 components of the fire triangle are present among the five factors.
· The nature of the fuel used.
· The concentration of oxygen
· The surface area of the fuel
· Temperature
· Presence of catalysts
Example 1 Relate how each of the first four factors listed above affect the rate at which wood is burned in a fireplace.
a. The nature of the
fuel used:
·
The amount of moisture
still present in wood slows the rate of combustion.
·
Certain types of wood
contain more resin or oils, and these increase the combustion rate.
·
Structurally, different
trees synthesize different fibers and these don't all burn at the same rate.
b. The concentration of
oxygen:
·
The higher the
concentration of oxygen, the faster wood will burn. This applies to all other
combustible materials.
c. The surface area of
the fuel:
·
If you take two
identical logs and convert one into wood chips, the intact one will burn a lot
slower. Because of its lower surface area, the intact log has less of its
molecules exposed to oxygen. This means that oxygen molecules will only collide
with a tiny proportion of the total available wood molecules. Without
collisions, no reaction will take place, and so low surface area dramatically
slows down the rate of combustion.
d. Temperature:
· Raising the temperature increases the kinetic energy of both oxygen and fuel molecules. This will lead to more overall collisions and to more effective collisions between the fuel and O2. Since more molecules are reacting in the same period of time this increases the rate of combustion.
e. Catalysts:
·
A catalyst is a
chemical that speeds up a reaction. (The catalyst, as we'll explore later, is
itself not consumed in the reaction.) So any chemical that makes it easier for oxygen
to take electrons away from wood will lower the activation energy and increase
the rate at which wood burns.
Exercises for
Rate of Combustion
1. Write a balanced equation for the combustion of pentane. Pentane is C5 H12. Identify the fuel and the oxide, and include heat on the appropriate side of the equation.
2. Draw a reaction profile in which H reactants = 100 kJ, the activation energy is 50 kJ, and DH = -25 kJ.
3. Which of the following is not absolutely necessary for combustion to take place?
A. a fuel B. oxygen
C. match D. a sufficiently high temperature
4. Carbon burns in air to produce CO2 and H2O. Under what conditions would you expect the fastest rate of combustion?
A. Chunks of carbon are heated and allowed to burn naturally in air.
B. Forcing hot air over the carbon as it burns.
C. Powdering the carbon and allowing it to burn naturally.
D. Powdering the carbon and forcing hot air over it.
5. How does the foam from a fire extinguisher help put out the fire?
6. A cook started a fire by forgetting about the oil he was warming. He then pulled the pan off the stove and threw baking soda in the pan.
Explain why the cook acted the way he did. Why did he not use water?
7. Olive oil has a lower kindling point than corn oil. Which one is the more practical cooking oil?
8. According to the manufacturer, the average rate of combustion of a certain
type of candle made of paraffin, C25H52 , is 8.33 x10-4 mol/min. This type of
candle is sold in four sizes: 25, 50, 75 or 100 g. You wish to use only one
candle of this type to provide 4 continuous hours of light. What is the smallest
one you can buy for this purpose ?
9. From a molecular point of view, why does a combustion reaction need to reach a kindling point before a fire starts?
10. From a molecular point of view, why does surface area increase the rate of a reaction?