|
Solutions to 2001 Monthly Questions
|
Solutions to 2001 Monthly Questions
The question was:
Imagine a sphere of calcium. If you slice it in half, how much faster will it react with water, provided that all other rate-affecting factors remain the same?
Solution: By slicing it in half, the two halves expose an extra two circles or an extra 2pr2 units of area for a total of 6pr2 units. Comparing that to the original surface area of a sphere, we have 1.5 times as much area, so the reaction should proceed 1.5 times as fast.
The question was:
Bases, those compounds that neutralize acids, feel slippery. Why are they slippery?
Solution:
In what is known as a saponification reaction, bases typically react with lipids to create fatty acids. Fatty acid molecules can be used as soaps because they have both a hydrophobic (water fearing) end that attaches itself to grease and a hydrophilic end that dissolves in water. When you get a base on your hands it reacts with the oils secreted by your skin to form fatty acids, which have a soapy feel to them.
The question was:
If you place your hand in hot water and then in a pan of room temperature water, you get a cold sensation. Take the other hand from a pan of cold water and place into that same room-temp pan, and you feel a warm sensation. What does that suggest about our nerves' ability to measure temperature?
Solution:
It seems that the nerves are not acting as a thermometer because they are relaying a different message to the brain even though the temperature is the same. Instead the nerves seem to be detecting a temperature change. The hand that was in hot water loses heat in the room-temp pan, so it feels cold. The hand that was in cold water gains heat in the room-temp pan, so it feels warm.
The question was:
Where can you easily see the following phenomenon at work, with approximately the colours shown?
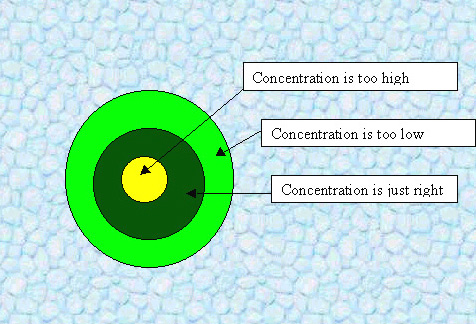
Solution:
If a dog has peed* in a lawn, the grass directly under the waste
gets an excessive dose of urea, killing the grass and rendering
it yellow. Within a certain radius, however, the concentration is just right
to encourage growth(after it gets converted to nitrates), so the grass becomes a deeper green. Too far from the
source of the nitrate, the grass is, although still alive, not as healthy.
(*At one point
I thought the phenomenon was due to poop, but soon after I became a dog-owner, I realized that urine is the cause.
Besides poop is usually scooped. )
From reading http://polk.uwex.edu/hort/lawnanddogs.html
I was also reminded that the reason dog-urine has a high concentration of urea is that dogs are carnivores.
A diet rich in proteins(full of nitrogen) means more nitrogenous wastes, which, in most mammals, are expelled in the form of urea.
Egg-laying mammals and birds have to dispose of nitrogenous wastes in the form of water-insoluble uric acid
because water-soluble urea would poison the food supply of the embryo inside and egg. Fish
excrete ammonia, which although more toxic, is cheaper to manufacture and becomes innocuous after dilution in
large bodies of water.

The question was:
Until recently, scientists had the false impression that CCA-treated wood [containing Cu, Cr and As(arsenic)] was safe in all circumstances. In the lab, they had soaked treated wood in water for various periods of time, analyzed the resulting solutions,and found them to have very low concentrations of arsenic. This implied that the As was safely bound to other chemicals in the wood.
But a guy named Stilwell working at the Connecticut Agricultural Experimental Station (see Bulletin of Environmental Contamination and Toxicology (1997) 58:22-29 ) decided to analyze the soil below people's treated-wood decks for arsenic. In many cases, he found alarming amounts of the toxic metal.
Why was there such a difference between the laboratory and field experiments? What related environmental problem did the lab scientists fail to take into account?
Solution:
In the lab scientists had used distilled water or other water with pH from 6 to 7.At these pH's only a small amount of As dissolves from the treated wood. In northeastern U.S. and Canada, acidic precipitation often has a pH<5. With this level of acidity much more As leaches out of decks and ends up in the soil.
The question was:
Identify the molecule from the following clues:
Solution:
The molecule is nitrogen monoxide(NO). For a good discussion of this molecule read "Molecules at an Exhibition" by John Emsley. It was published in 1998 by Oxford University Press and is available at the St. Laurent public library. The book was published just before Viagra appeared on the market. Emsley did however speculate on the eventual availability of such a drug.
The question was:
Identify the molecule and the food that contains it from the following clues:
Solution:
The molecule is phenylethylamine (PEA) and it is found not in peas but in cocoa beans and hence in chocolate. For a good discussion of this molecule read "Molecules at an Exhibition" by John Emsley(p 3-6). It was published in 1998 by Oxford University Press and is available at the St. Laurent public library.
The question was:
Recently, I removed the treated wood borders from my garden. In fact, in the U.S., the Environmental Protection Agency does not recommend CCA treated wood in the vicinity of edible plants. Why?
Solution: CCA-treated wood contains Cu(copper), chromium(Cr) and arsenic(As). Arsenic is in large doses poisonous, and in smaller doses it is carcinogenic(cancer-causing). These chemicals prevent fungi, bacteria and insects from breaking down treated wood but are slowly released into the soil. Eventually, they concentrate in plants, especially in roots, and to a lesser degree in leaves. When I removed the treated wood from my garden, I noticed that the flat boards that I would walk on had become an under-carpet for roots, so it is wrong to assume that water and gravity will keep the roots out of reach with the toxins. For more details on David Stilwell's research see Health and Welfare Canada
The question was:
Literally, why does the grass often seem greener on the other side?
Solution:
When looking at his own grass, our neighbour has more of an aerial view. He sees the top of the grass blades, so he’s seeing only a small portion of their total green area, and the bare or yellow spots are more obvious. When observing your lawn, he sees your grass lengthwise: more grass-surface is exposed to his viewpoint, concealing the bare spots, and leading to the false conclusion that the grass is greener on your side.
The question was:
You want to cook an egg in a new gadget that uses no water. It just contains the mold of a half-egg cast in stainless steel with a cover containing the other half of the mold that closes over the egg. Aside from the fact that eggs vary in size, for what other reason will this gadget not work as well as water?
Solution:
Because steel has a lower heat capacity than water[about 0.4 versus 4.19 J/(gC)],the bottom part of the mold would warm up too fast and its top would cool off too fast. More importantly, because of steel's high melting point, the temperature would keep increasing past the boiling point of water. But once water boils, if pressure remains constant, the water stays at about 100 C, allowing for a far more controlled cooking of the egg.
The question was:
Using units, show that a volt multiplied by an amp is indeed a watt.
Solution:
A volt is 1 J/C because it is related to the amount of energy lost by electrons as they come across a resistance. 1 amp is the amount of charge that flows by every second, more precisely 1 C/s. If we multiply a volt by an amp, we get (J/C)(C/s) = J/s. Energy in joules used per second is a watt, a unit of power.
The question was:
A student collects hydrogen gas by attaching one end of a rubber tube to a flask containing acid and magnesium and the other end to a bottle filled with water. Gradually, gas displaces water from the bottle. Depending on the amount of acid in the flask, the student will get varying percentages of hydrogen in the collecting bottle. Regardless of the amount of acid, the first bottle will never be 100% hydrogen. In fact, even the fifth bottle collected, although purer, will still not be 100% hydrogen. What's going on?
Solution:
Initially the air from the tube and from the reaction flask get mixed with the emerging hydrogen. But even as the continuous supply of hydrogen displaces the air, it never becomes pure because it is collected over water. H2O vapour mixes with the hydrogen; the amount of water vapour is directly proportional to temperature.