Solutions to 2006 Monthly Questions

Solution to January 2006 Question of the Month
The question was:
The Standard Model in physics, which was perfected in the early 1980's, accounts for why only certain
isotopes are stable; it explains what keeps atoms and molecules together, and why the sun shines.
But of course it has its limitations. Which of the following cannot be explained by the Standard Model?
- Why the universe is expanding at an accelerating rate, in spite of gravity.
- Why the universe does not contain equal parts of matter and antimatter.
- How gravity is connected to the other fundamental forces: electromagnetic, weak and strong forces.
Solution:
None of the four can be explained by the Standard model. The exciting thing is that as a result
of this ignorance, we are on the eve of a revolutionary theory in physics.
Solution to February 2006 Question of the Month
The question was:
Spiritually, the human body may be full of aspirations,
but from the strict point of view of mass, the human body is mostly H2O.
Its tissue is mostly protein with varying amounts of fat and both contribute
more hydrogen and oxygen, as well as carbon. Proteins and genetic material add nitrogen to the heterogeneous mixture of the genus Homo.
DNA, RNA and bones also contain phosphorus, and of course there is calcium thoughout our cells and skeleton. Finally there would be no movement without ions of sodium,
potassium and chloride, no respiration without iron, and many enzymes depend on trace
amounts of various other metallic ions.
Here's a summary:
| ELEMENT |
Mass%in the human body |
| O |
65 |
| C |
18 |
| H |
10 |
| N |
3 |
| Ca |
1.5 |
| P |
1.2 |
| K |
0.2 |
| Cl |
0.2 |
| Na |
0.1 |
| Mg |
0.05 |
| Fe |
< 0.05 |
But mass is not really a fair comparison because the oxygen atom is 16 times heavier than hydrogen. The fact that there is only 6.5 times as much oxygen, mass-wise, suggests that hydrogen atoms are actually more common than oxygen atoms in the human body (not surprising; think of the ratio in H2O)
The question is this. How do you convert mass percent into percent of atoms in the human body?
Solution:
Consider the amounts for each element in 100 g . Convert each into moles.
Then with the total number of moles, find the mole% of each, which will be equal to
the atom% for each element in the human body:
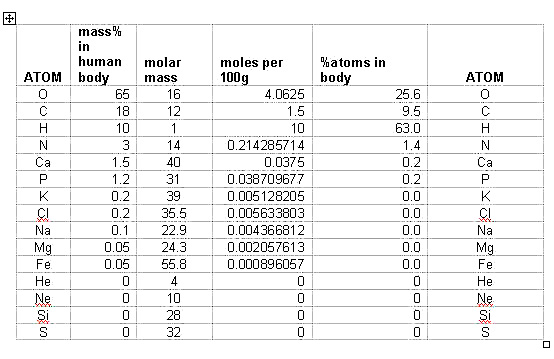
The most abundant elements in our body then are H, O, C, N, P and Ca.
In the universe the order is H, He, O, C, N, Si and Mg. The differences are that the
inert helium cannot be used by life forms. Silicon is an important part of the crust that
harbors life, but the Si atom cannot catenate with itself and is therefore not as useful
as carbon. Phosphorus, interestingly, is rare in the universe but more common to life.
Solution to March 2006 Question of the Month
The question was:
A parasitic plant known as the dwarf mistletoe uses
water pressure to project its seeds as far as 15 m away from the source. Someone measured
an initial velocity of 100 km/h. Assuming that the optimal angle
of 45o is used, does air friction play a significant role in reducing the
distance traveled by the seeds?
Solution:
A small object has a high surface area to volume ratio, so air friction
will play an important role in slowing down the seed. Calculations
reveal that, without friction, a 44 km/h initial velocity would suffice to launch the seed
15 m. Conversely, a 100 km/h initial velocity, without air friction, would deposit
the seed at an astounding distance of almost 79 m from the source.(Notice that distance
traveled is
proportional to the square of the projectile velocity.)
Solution to April 2006 Question of the Month
The question was:
Background Information
Last month CBC's Quirks and Quarks interviewed Margaret Turnbull,
an astronomer at the Carnegie Institution of Washington.
Starting with 120 000 stars from the Hipparcos catalogue,
she applied a set of criteria to look for stars that could support life.
They had to live long enough; they could not be too variable in brightness;
they preferably had no companions or were part of binaries that would not
interfere with a star's habitable zone. After filtering through the original list,
she was able to present the SETI Institute (search for extra terrestrial life)
with a list of 19 000 stars . Her favorite star from the list is 18 Sco, a solar twin.
Here's why:
It is a good candidate to have planetary system
It has the same age as the sun;
has the same metal content as our sun;
could have a planetary system
shows very little variability;
it's not part of binary system of stars
It has an absolute magnitude(real indication of brightness) of 4.775
(our sun's = 4.8)
Since the radio program left me with unanswered questions,
I decided to carry out my own interview with Margaret Turnbull.
Actually the whole thing was done through email, but the task was accomplished nevertheless.
Here's the exchange:
LHA: What kind of data did you use to conclude that 18 Sco and our sun
have a similar metal content?(Metal-rich stars like our sun are younger and more likely
to form planets.)
M. Turnbull: I use spectroscopy data. The spectrum of a star shows the blackbody
emission corresponding to the star's temperature, plus narrow
absorption features at wavelengths where the various elements have
transitions. You probably talk all about that in your chemistry class,
but Cecelia Payne was the first person who figured out how to use those
spectra to calculate how much of each element is present in stars.
LHA: From the apparent and absolute magnitudes of 18 Sco, I calculated a distance
of about 42 light years to earth. Is that correct?
M. Turnbull: That sounds about right (but I don't have things like that memorized).
The equation to use is m - M = 5 log (d) -5, where m is the apparent
magnitude, M is the absolute magnitude, and "d" is in parsecs. = Then after you
solve for d, you can convert from parsecs to light years.
Alternatively, you can use parallax data to convert to parsecs by
dividing 1000 by the parallax in milli-arcseconds. (or dividing 1 by
the parallax in arcseconds.)
Let's check again(you'll see, I was off a bit:
m - M = 5 log (d) -5
(5.510 - 4.775 + 5) / 5 = log d
1.147 = log d
d = 10 ^(1.147) = 14. 03 parsecs
14.03 parsecs(3.26 light-years /parsec) = 46 light years
LHA: What other stars made it to the "short list" that you referred to
on Quirks and Quarks?
M. Turnbull: I think I talked about 18 Sco and 51 Peg and beta CVn. Some other good
ones are epsilon Eridani, Chara, alpha Cen A and B, 37 Gem, 61 Vir, eta
Cas A, 47 UMa, tau Ceti, epsilon Indi A, 82 Eri, and delta Pav. Most
of those names are shorthand for the constellation and the brightness
or location of the star within that constellation. Astronomy
nomenclature is a long story in itself!
I appreciate your interest in this topic! Looking for aliens is a fun
way to learn all different kinds of science--at least it has been for
me!
Solution to May 2006 Question of the Month

The question was:
The mineral mangan neptunite has the empirical formula K2Na4Li2Mn3Fe2+Ti4Si16O48
but it really consists of seven oxides, all of which are
binary compounds. Can you give formulas for each of the oxides? We expect you
to get the answer from a basic knowledge of the periodic table and from a bit
of logic, not from knowing geology.
Solution:
In the compound
K2Na4Li2Mn3Fe2+Ti4Si16O48
we find:
K+, O-2 = K2O
Na+ , O-2 =
Na2O
Li+, O-2 = Li2O
Fe+2(given as such) = FeO
SiO2 (same ratio as family member carbon)
To figure out Mn and Tiís charges, we apply some logic:
So for two K and two Li we need a total of only 2 oxygens.
Four sodiums require 2 oxygens. Sixteen Siís(see formula) need 32 oxygens. One Fe+2 needs 1
oxygen. There are 48 oxygens in all in manganís formula. So far we have accounted for 37. The remaining 11 oxygens bond to 3 Mnís and 4 titaniums.
Let x = Mnís charge
Let y = Tiís charge
3x + 4y = 22
x = (22 Ė 4y)/3
From trial and error we see that the only whole numbers that
satisfy the above equation are
y = 1 and x = 6.(but 6 is too high an oxidation # for Mn in a mineral)
y = 4 and x = 2.
So Mn+2 and O-2 = MnO.
Three Mnís require 3 oxygens, which leaves (11-3 =) 8 for titanium, which is
exactly what four Ti+4 need.
Ti +4, O-2 = TiO2.
Solution to June 2006 Question of the Month
The question was:
Fe3O4 is a compound that comes with surprises. It seems like a
simple ionic compound with a common metal(iron) bonded to the non metal oxygen.
Yet unlike most ionic compounds, it conducts electricity in the solid state. It is also magnetic
and the main ingredient of the first discovered magnet: lodestone. Since oxygen
has an oxidation number of -2, the Fe seems to have an oxidation state of +8/3. But the
fractional result is based on a false assumption: that all the irons are identically charged.
Given that they are actually of two different oxidation states, what possible charges does iron have in
Fe3O4?
Solution:
Since there four oxygens, the three irons have a total charge of +8.
let x = charge of one iron type
y = charge of the other type
let a = number of iron types of charge x
let b = number of iron types of charge y
a + b = 3 (there are three irons);
a and b must be 2 and 1; the only non-zero whole numbers that add up to 3.
2x + 1y = 8
y = 8 - 2x.
Whole number solutions are:
x = 1; y = 6
x = 2; y = 4
x = 3; y = 2
Since oxidation of states of 4 and 6 don't exist for iron, there must
be two +3 iron atoms for every +2 iron in Fe3O4 .
Electrons can jump from one type of iron ion to the other,
possibly accounting for its unusual
conductivity.
Fe3O4 is an example of a spinel ,
a class of minerals which crystallize in the isometric system
with an octahedral habit.
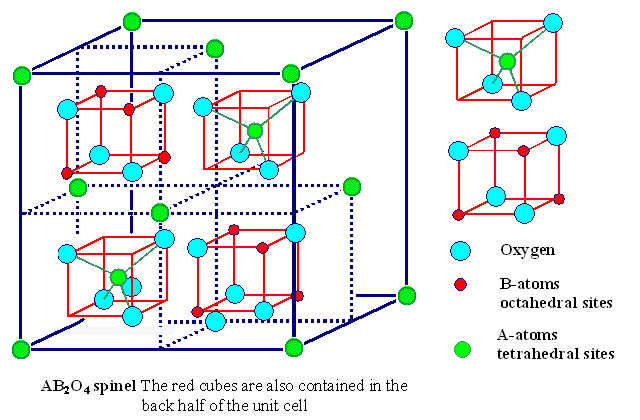
diagram from http://www.techfak.uni-kiel.de/matwis/amat/def_en/kap_2/illustr/spinel.gif
The general formula is as (X2+)(Y3+)2(O2-)4,
with X representing a divalent cation
and Y a trivalent cation. If you're confused by the ratio of atoms in the diagram,
note that the four red cubes and the two green sandwiched atoms account for
16 blue spheres (oxygen), 8 red spheres(Fe+3) and 4 green ones
(Fe+2). 16:(8+4) = 4:3 overall ratio of O to Fe, including the 8:4 or 2:1
ratio of +3 to +2 ions.
Solution to July 2006 Question of the Month
The question was:
The Earth is believed to be 4.54 billion years, give or take about 70 million years.
After many way-off attempts by other scientists in the late 19th and early 20th centuries,
Clair Patterson's hard work paid off, and his estimate is still believed to be accurate today.
What kind of experiments have led to this value, and is a formula used to turn the
data into an age?
Solution:
Solution to August 2006 Question of the Month
The question was:
Something I read in a Joe Schwartz book reminded me that over a year ago student
David D. mentioned that ants are the main source of acid rain. That is not exactly accurate, but it is
partly true, and finally, David, if you're still out there, as promised, I finally looked into it!
What is the connection between ants and acid rain?
Solution:
Ants, especially wood ants, secrete formic acid in defending themselves.
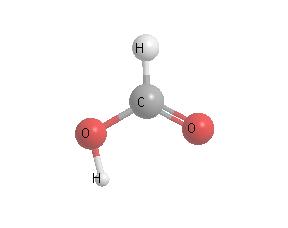 In the tropics, which boasts the planet's greatest concentration of such insects,
the atmosphere ends up with a fair amount of the acid. So in isolated areas,
formic acid is the main component of acid rain. In the rest of the world, however,the main acids in polluted rain are
sulfuric and nitric acids, which are emitted by industry, vehicles and volcanoes.
In the tropics, which boasts the planet's greatest concentration of such insects,
the atmosphere ends up with a fair amount of the acid. So in isolated areas,
formic acid is the main component of acid rain. In the rest of the world, however,the main acids in polluted rain are
sulfuric and nitric acids, which are emitted by industry, vehicles and volcanoes.
The amount of formic acid released by ants is greater than the world's entire industrial output.
Formic acid is produced commercially because it is used in textile dyeing and finishing, leather tanning
and, in Europe especially, as a forage crop preservative.
For those of you who may lose sleep if I don't reveal how formic acid is made by companies like BASF:
CH3OH(methanol) + CO (carbon monoxide) => HCO2CH3(methyl formate)
HCO2CH3(methyl formate) + H2O => HCO2H(formic acid) + CH3OH(methanol)
References:
Schwartz, Joseph. Dr. Joe and What You Didn't Know.ECW Press. 2003
Monastersky, Richard. Science News. 5/30/1987
http://en.wikipedia.org/wiki/Formic_acid
http://www2.basf.de/en/intermed/news/topstory/archiv/ameisensaeure.htm?id=V00-hopwV8wPLbw23aA
Solution to September 2006 Question of the Month
The question was:
The body of a 70 kg man contains about 5.6 L of blood, which is 92 % water.
His muscles, which account for 40% of his body mass, are 80% water.
The rest of his body (organs, bones and tissues) is on average about 50% water.
According to these figures, what percent of the human body is water?
Solution:
The density of blood is similar to that of water, about 1.0 kg/L, so 5.6 litres is approximately
5.6 kg
| blood |
0.92(5.6 kg) |
5.152 kg |
| muscle |
0.40(70 kg)(0.80) |
22.4 kg |
| miscellaneous |
0.50[70kg -0.40(70kg)]-5.152 kg |
18.425 kg |
| total |
|
45.977 kg |
45.977/70kg *100% = 66%. Using significant figures, our answer must be rounded to 70% water.
References
Chernow and al.(editors).Columbia Encyclopedia. 1993
Berkow, Robert(editor)The Merck Manual 16th edition. 1992
Solution to October 2006 Question of the Month
When water in food absorbs microwaves, H2O molecules gain rotational kinetic energy, and it is
this energy that eventually heats the rest of the food molecules and the dish itself.
Johnny has a strange appetite for candle wax. He places a candle in a strong microwave
and turns it on for 30 seconds.
The question was:
Which of the following predictions is logically consistent with the above information?
(A) Oxygen molecules will translate fast enough to light the candle.
(B) There are not enough oxygen molecules in the microwave to light the candle.
(C) The wax will absorb microwaves and the extra vibrations will melt the candle.
(D) Nothing will happen.
Solution:
D. There is no water in candle wax, so the candle will not
be heated. Try it for 30 seconds, and you will see.
To understand how the magnetron in a microwave gets electrons to release energy, see
this link
Solution to November 2006 Question of the Month
The question was:
What substance becomes deadly when neutralized?
Solution:
The question is a little deceiving in its wording. Whe we read "neutralized"
we think of acid-base neutralizations or of a euphemism for destruction.
But the answer is the ion fluoride (F-1), which if "neutralized" or rendered neutral becomes
the deadly gas fluorine. Fluoride itself is dangerous: 5 to 10 grams will kill the average sized adult( that works out to about 100 mg per kg).
In lower concentrations,however, fluoride can help strengthen teeth. It is also naturally found in minerals and in water.
Neutral fluorine is lethal at a concentration of 170 mg per L of air.
But even at lower concentrations, fluorine penetrates deeply into body tissues and if it is
not neutralized it will continue to do damage.
Berkow, Robert(editor)The Merck Manual 16th edition. 1992
or
Solution to December 2006 Question of the Month
The question was:
From analyzing ancient skeletons, paleoepidemiologists have been able to
determine if people at the time had died of tuberculosis.
How can they tell? Do they identify bacterial spores that have survived
thousands of years, or is there another and more subtle component that acts as evidence?
Solution:
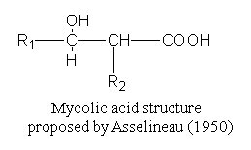
Paleoepidemiologists rely on mycolic acids, which are long fatty acids found in the cell
walls of a group of bacteria known as mycolata. The latter includes the species Mycobacterium
tuberculosis, which causes tuberculosis.
Mycolic acids are comprised of a shorter beta-hydroxy chain (including C-OH group with R1)
with a longer alpha-alkyl side chain(R2).
Each molecule contains between 60 and 90 carbon atoms.
The exact number of carbons varies from species to species and it is this characteristic
that acts as a fingerprint for the disease-causing type of bacteria.
References:
Wikipedia
cyberlipid.org
BBC
elementy.ru
Jones, Martin. The Molecule Hunt. Penguin UK. 2002
Page Maintained by E.Uva
Comments: euva@retired.ca
euva@prof.emsb.qc.ca
Copyright ©2011











 In the tropics, which boasts the planet's greatest concentration of such insects,
the atmosphere ends up with a fair amount of the acid. So in isolated areas,
formic acid is the main component of acid rain. In the rest of the world, however,the main acids in polluted rain are
sulfuric and nitric acids, which are emitted by industry, vehicles and volcanoes.
In the tropics, which boasts the planet's greatest concentration of such insects,
the atmosphere ends up with a fair amount of the acid. So in isolated areas,
formic acid is the main component of acid rain. In the rest of the world, however,the main acids in polluted rain are
sulfuric and nitric acids, which are emitted by industry, vehicles and volcanoes.